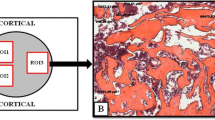
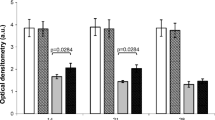
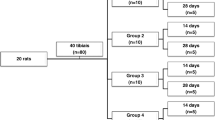
Abstract
Diabetes mellitus (DM) leads to a decrease in bone mass and increase the risk of osteoporosis and in this context, many treatments have shown to accelerate bone metabolism. It seems that low-level laser therapy (LLLT) is able of stimulating osteoblast activity and produced increased biomechanical properties. However, its effects on bone in diabetic rats are not fully elucidated. The aim of this study was to evaluate the effects of LLLT on bone formation, immunoexpression of osteogenic factors, biomechanical properties and densitometric parameters in diabetic rats. Thirty male Wistar rats were randomly distributed into three experimental groups: control group, diabetic group, and laser-treated diabetic group. DM was induced by streptozotocin (STZ) and after 1 week laser treatment started. An 830-nm laser was used, performed for 18 sessions, during 6 weeks. At the end of the experiment, animals were euthanized and tibias and femurs were defleshed for analysis. Extensive resorptive areas as a result of osteoclasts activity were noticed in DG when compared to control. Laser-treated animals showed an increased cortical area. The immunohistochemical analysis revealed that LLLT produced an increased RUNX-2 expression compared to other groups. Similar RANK-L immunoexpression was observed for all experimental groups. In addition, laser irradiation produced a statistically increase in fracture force, bone mineral content (BMC) and bone mineral density compared to DG. The results of this study indicate that the STZ model was efficient in inducing DM 1 and producing a decrease in cortical diameter, biomechanical properties and in densitometric variables. In addition, it seems that LLLT stimulated bone metabolism, decreased resorptive areas, increased RUNX-2 expression, cortical area, fracture force, BMD, and BMC. Further studies should be developed to provide additional information concerning the mechanisms of action of laser therapy in diabetic bone in experimental and clinical trials.






Similar content being viewed by others
References
Einhorn TA, Boskey AL, Gundberg CM, Vigorita VJ, Devlin VJ, Beyer MM (1988) The mineral and properties of bone in chronic experimental diabetes. J Orthop Res 6(3):317–323
Suzuki K, Miyakoshi N, Tsuchida T, Kasukawa Y, Sato K, Itoi E (2003) Effects of combined treatment of insulin and human parathyroid hormone(1–34) on cancellous bone mass and structure in streptozotocin-induced diabetic rats. Bone 33(1):108–114
Mathiassen B, Nielsen S, Johansen JS, Hartwell D, Ditzel J, Rødbro P, Christiansen C (1990) Long-term bone loss in insulin-dependent diabetic patients with microvascular complications. J Diabetes Complicat 4(4):145–149
Thrailkill KM, Lumpkin CK Jr, Bunn RC, Kemp SF, Fowlkes JL (2005) Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues. Am J Physiol Endocrinol Metab 289(5):e735–e745
Wongdee K, Charoenphandhu N (2011) Osteoporosis in diabetes mellitus: possible cellular and molecular mechanisms. World J Diabetes 2(3):41–48
Blakytny R, Spraul M, Jude EB (2011) Review: the diabetic bone: a cellular and molecular perspective. Int J Low Extrem Wounds 10(1):16–32
Expert Commitee on the Diagnosis and Classification of Diabetes Mellitus, Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26(11):3160–3167
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15(7):539–553
Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5):1047–1053
American Diabetes Association, Hogan P, Dall T, Nikolov P (2003) Economic costs of diabetes in the US in 2002. Diabetes Care 26(3):917–932
Bashardoust Tajali S, Macdermid JC, Houghton P, Grewal R (2010) Effects of low power laser irradiation on bone healing in animals: a meta-analysis. J Orthop Surg Res 5:1
Bashardoust Tajali S, Ebrahimi E, Kazemi S, Bayat M, Azari A, Azordegan F, Kamali M, Hoseinian M (2003) Effects of He–Ne laser irradiation on osteosynthesis. Osteo Trauma Care 11:17–20
Garavello-Freitas I, Baranauskas V, Joazeiro PP, Padovani CR, Dal Pai-Silva M, Da Cruz-Höfling MA (2003) Low-power laser irradiation improves histomorphometrical parameters and bone matrix organization during tibia wound healing in rats. J Photochem Photobiol 70(2):81–89
Khadra M, Kasem N, Haanas HR, Ellingsen JE, Lyngstades SP (2004) Enhancement of bone formation in rat calvarial bone defects using low-level laser therapy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 97(6):693–700
Bayat M, Abdi S, Javadieh S, Mohsenifar Z, Rashid MR (2009) The effects of low-level laser therapy on bone in diabetic and nondiabetic rats. Photomed Laser Surg 27(5):703–708
Luger EJ, Rochkind S, Wollman Y, Kogan G, Dekel S (1998) Effect of low-power laser irradiation on the mechanical properties of bone fracture healing in rats. Lasers Surg Med 22(2):97–102
Freitas IGF, Baranauskas V, Cruz-Höfling MA (2000) Laser effects on osteogenesis. Appl Surface Sci 154–155:548–554
Abdi S, Bayat M, Javadieh F, Mohsenifar Z, Rezaie F, Bayat M (2009) The effects of helium-neon light therapy on healing of partial osteotomy of the tibia in streptozotocin induced diabetic rats. Photomed Laser Surg 27(6):907–912
Reddy GK, Stehno-Bittel L, Enwemeka CS (2001) Laser photostimulation accelerates wound healing in diabetic rat. Wound Rep Reg 9(3):248–255
Yu W, Naim JO, Lanzafame RJ (1997) Effects of photostimulation on wound healing in diabetic mice. Lasers Surg Med 20(1):56–63
Coombe AR, Ho CT, Darendeliler MA, Hunter N, Philips JR, Chapple CC, Yum LW (2001) The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res 4(1):3–14
Javadieh F, Bayat M, Abdi S, Mohsenifar Z, Razi S (2009) The effects of infrared low-level laser therapy on healing of partial osteotomy of tibia in streptozotocin-induced diabetic rats. Photomed Laser Surg 27(4):641–646
He H, Liu R, Desta T, Leone C, Gerstenfeld LC, Graves DT (2004) Diabtes causes decreased osteoclastogenesis, reduced bone formation, and enhanced apoptosis of osteoblastic cells in bacteria stimulated bone loss. Endrocrinology 145(1):447–452
Renno AC, De Moura FM, Dos Santos NS, Tirico RP, Bossini PS, Parizotto NA (2006) Effect of 830-nm laser light on preventing bone loss after ovariectomy. Photomed Laser Surg 24(5):642–645
Medalha CC, Amorim BO, Ferreira JM, Oliveira P, Pereira RM, Tim C, Lirani-Galvão AP, Da Silva OL, Renno AC (2010) Comparison of the effects of electrical field stimulation and low-level laser therapy on bone loss in spinal cord-injured rats. Photomed Laser Surg 28(5):669–674
Anandarajah AP (2009) Role of RANKL in bone diseases. Trends Endocrinol Metab 20(2):88–94
Lozano D, Fernández-de-Castro L, Portal-Núñez S, López-Herradón A, Dapía S, Gómez-Barrena E, Esbrit P (2011) The C-terminal fragment of parathyroid hormone-related peptide promotes bone formation in diabetic mice with low-turnover osteopaenia. Br J Pharmacol 162(6):1424–1438
Cornish J, Callon KE, Reid IR (1996) Insulin increases histomorphometric indices of bone formation in vivo. Calcif Tissue Int 59(6):492–495
Huang S, Kaw M, Harris MT, Ebraheim N, Mclnerney MF, Najjar SM, Lecka-Czernik B (2010) Decreased osteoclastogenesis and high bone mass in mice with impaired insulin clearance due to liver-specific inactivation to CEACAM1. Bone 46(4):1138–1145
Higgins TF, Johnson BD (2010) Effect of exogenous IGF-1 on chondrocyte apoptosis in a rabbit intraarticular osteotomy model. J Orthop Res 28(1):125–130
Fávaro-Pípi E, Ribeiro DA, Ribeiro JU, Bossini P, Oliveira P, Parizotto NA, Tim C, De Araújo HS and Renno AC (2011) Low-level laser therapy induces differential expression of osteogenic genes during bone repair in rats
Pires-Oliveira DA, De Oliveira RF, Zangaro RA, Soares CP (2008) Evaluation of low-level laser therapy of osteoblastic cells. Photomed Laser Surg 26(4):401–404
Stein A, Benayahu D, Maltz L, Oron U (2005) Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed Laser Surg 23(2):161–166
Fernandes KR, Ribeiro DA, Rodrigues NC, Tim C, Santos AA, Parizotto NA, De Araujo HS, Driusso P, Rennó AC (2013) Effects of low-level laser therapy on the expression of osteogenic genes related in the initial stages of bone defects in rats. J Biomed Opt 18(3):038002
Yaoita H, Orimo H, Shirai Y, Shimada T (2000) Expression of bone morphogenetic proteins and rat distal-less homolog genes following rat femoral fracture. J Bone Miner Metab 18(2):63–70
Fávaro-Pípi E, Feitosa SM, Ribeiro DA, Bossini P, Oliveira P, Parizotto NA, Renno AC (2010) Comparative study of the effects of low-intensity pulsed ultrasound and low-level laser therapy on bone defects in tibias of rats. Lasers Med Sci 25(5):727–732
Komori T (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339(1):189–195
Bossini PS, Rennó AC, Ribeiro DA, Fangel R, Ribeiro AC, Lahoz Mde A, Parizotto NA (2012) Low level laser therapy (830nm) improves bone repair in osteoporotic rats: similar outcomes at two different dosages. Exp Gerontol 47(2):136–142
Kearns AE, Khosla S, Kostenuik PJ (2008) Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev 29(2):155–192
Nyman JS, Even JL, Jo CH, Herbert EG, Murry MR, Cockrell GE, Wahl EC, Bunn RC, Lumpkin CK Jr, Fowlkes JL, Thrailkill KM (2011) Increasing duration of type 1 diabetes perturbs the strength-structure relationship and increases brittleness of bone. Bone 48(4):733–740
Nissan J, Assif D, Gros MD, Yaffe A, Binderman I (2006) Effect of low intensity laser irradiation on surgically created bony defect in rats. J Oral Rehabil 33(8):619–924
Akyol UK, Güngörmüş M (2010) Effect of biostimulation on healing of bone defects in diabetic rats. Photomed Laser Surg 28(3):411–416
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patrocínio-Silva, T.L., de Souza, A.M.F., Goulart, R.L. et al. The effects of low-level laser irradiation on bone tissue in diabetic rats. Lasers Med Sci 29, 1357–1364 (2014). https://doi.org/10.1007/s10103-013-1418-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-013-1418-y