Abstract
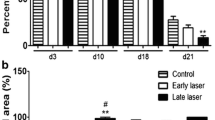
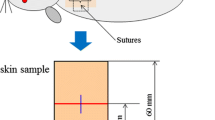
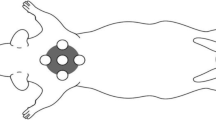
This study explored the inhibitory effect of the high-power helium–neon (He–Ne) laser on the growth of scars post trauma. For the in vitro study, human wound fibroblasts were exposed to the high-power He–Ne laser for 30 min, once per day with different power densities (10, 50, 100, and 150 mW/cm2). After 3 days of repeated irradiation with the He–Ne laser, fibroblast proliferation and collagen synthesis were evaluated. For in vivo evaluation, a wounded animal model of hypertrophic scar formation was established. At postoperative day 21, the high-power He–Ne laser irradiation (output power 120 mW, 6 mm in diameter, 30 min each session, every other day) was performed on 20 scars. At postoperative day 35, the hydroxyproline content, apoptosis rate, PCNA protein expression and FADD mRNA level were assessed. The in vitro study showed that the irradiation group that received the power densities of 100 and 150 mW/cm2 showed decreases in the cell proliferation index, increases in the percentage of cells in the G0/G1 phase, and decreases in collagen synthesis and type I procollagen gene expression. In the in vivo animal studies, regions exposed to He–Ne irradiation showed a significant decrease in scar thickness as well as decreases in hydroxyproline levels and PCNA protein expression. Results from the in vitro and in vivo studies suggest that repeated irradiation with a He–Ne laser at certain power densities inhibits fibroblast proliferation and collagen synthesis, thereby inhibits the growth of hypertrophic scars.











Similar content being viewed by others
References
Shu B (ed) (2010) Trauma rehabilitation, 1st edn. People's Health Press, Beijing
Har-Shai Y, Amar M, Sabo E (2003) Intralesional cryotherapy for enhancing the involution of hypertrophic scars and keloids. Plast Recons Surg 111:1841–1852
Alster TS (1997) Laser treatment of hypertrophic scars, keloids and striae. Dermatol Clin 15:419–429
Goldman MP, Fitzpatrick RE (1995) Laser treatment of scars. Dermatol Surg 21:685–687
Breugel H, Bar PRD (1992) Power density and exposure time of He–Ne laser irradiation are more important than total energy dose in photo-biomodulation of human fibroblasts in vitro. Lasers Surg Med 12:528–537
Anderson RR, Parrish JA (1981) The optics of human skin. J Invest Dermatol 77:13–19
Hu WP, Wang JJ, Yu CL, Lan CCE, Chen GS, Yu HS (2007) Helium–neon laser irradiation stimulates cell proliferation through photostimulatory effects in mitochondria. J Invest Dermatol 127:2048–2057
Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, Herman IM, Hamblin MR (2007) Low-level light stimulates excisional wound healing in mice. Lasers Surg Med 39:706–715
Pinfildi CE, Liebano RE, Hochman BS, Ferreira LM (2005) Helium-neon laser in viability of random skin flap in rats. Laser Surg Med 37:74–77
Basford FR (1989) Low-energy therapy: controversies and new research findings. Lasers Surg Med 9:1–5
Gross AJ, Jelkmann W (1990) Helium-neon laser irradiation inhibits the growth of kidney epithelial cells in culture. Lasers Surg Med 10:40–44
Shu B, Wu ZY, Hao LL, Zeng DF, Fen GR, Lin YH (2002) Experimental study on He–Ne laser irradiation to inhibit scar fibroblast growth in culture. Chin J Trauma 5:246–249
Baldwin HE (2007) Keloids and hypertrophic scars: basic and clinical dermatology, 1st edn. CRC, Florida
Davis JM (2002) Basic cell culture, 2nd edn. Oxford University Press, New York
Sambrook J, Russell DW (2001) Molecular cloning: a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Zhang LQ, Loato M, Munona P (1994) Normal and hypertrophic scars: quantification and localization of messenger RNAs for type I, III and IV collagens. Br J Dermatol 130:453–459
Morris DE, Wu L, Zhao LL, Bolton L, Roth SI, Ladin DA, Mustoe TA (1997) Acute and chronic animal models for excessive dermal scarring: quantitative studies. Plast Reconstr Surg 100:674–681
Silveira PC, Silva LA, Freitas TP, Latini A, Pinho RA (2011) Effects of low-power laser irradiation (LPLI) at different wavelengths and doses on oxidative stress and fibrogenesis parameters in an animal model of wound healing. Lasers Med Sci 26:125–131
Pereira AN, Eduardo CP, Matson E, Marques MM (2002) Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts. Lasers Surg Med 31:263–267
Kreisler M, Christoffers AB, Al-Haj H, Willershausen B, d’Hoedt B (2003) Low level 809-nm diode laser-induced in vitro stimulation of the proliferation of human gingival fibroblasts. Lasers Surg Med 30:365–369
Marques MM, Pereira AN, Fujihara NA, Nogueira FN, Eduardo CP (2004) Effect of low-power laser irradiation on protein synthesis and ultrastructure of human gingival fibroblasts. Lasers Surg Med 34:260–265
Azevedo LH, Eduardo FP, Moreira MS, Eduardo CP, Marques MM (2006) Influence of different power densities of LILT on cultured human fibroblast growth: a pilot study. Lasers Med Sci 21:86–89
Karu TI, Pyatibrat L, Kalendo G (1995) Irradiation with He–Ne laser increases ATP level in cells cultivated in vitro. J Photochem Photobiol B 27:219–223
Yu HS, Wu CS, Yu CL, Kao YH, Chiou MH (2003) Helium–neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo. J Invest Dermatol 1:56–68
Pastore D, Greco M, Passarella S (2000) Specific helium–neon laser sensitivity of the purified cytochrome c oxidase. Int J Radiat Biol 76:863–870
Serarslan G, Altug E, Kontas T, Atik E, Avci G (2007) Caffeic acid phenethyl ester accelerates cutaneous wound healing in a rat model and decreases oxidative stress. Clin Exp Dermatol 32:709–715
Elwakil TF (2007) An in-vivo experimental evaluation of He–Ne laser photostimulation in healing Achilles tendons. Lasers Med Sci 22:53–59
Souza TOF, Mesquita DA, Ferrari RAM, Pinto DS, Correa L, Bussadori SK, Fernandes KPS, Martins MD (2011) Phototherapy with low-level laser affects the remodeling of type I and III collagen in skeletal muscle repair. Lasers Med Sci 26:803–814
Mesquita-Ferrari RA, Martins MD, Silva JA, Silva TD, Piovesan RF, Pavesi VCS, Bussadori SK, Fernandes KPS (2011) Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med Sci 26:335–340
Desmoulière A, Redard M, Darby I, Gabbiani G (1995) Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol 146:56–66
Pourreau-Schneider N, Ahmed A, Soudry M, Jacquemier J, Kopp F, Franquin JC, Martin PM (1990) Helium–neon laser treatment transforms fibroblasts into myofibroblasts. Am J Pathol 137:171–178
Medrado ARAP, Pugliese LS, Reis SR, Andrade ZA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32:239–244
Darby I, Skalli O, Gabbiani G (1990) α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63:21–29
Acknowledgments
The authors are grateful to the Daping Hospital for Third Military Medical University to provide funding for research. We thank our colleagues and students at Daping Hospital of Third Military Medical University who assisted with the intellectual and technical development of this research.
Author disclosure statement
The authors declare that there are no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Bin Shu and Guo-Xin Ni have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Shu, B., Ni, GX., Zhang, LY. et al. High-power helium–neon laser irradiation inhibits the growth of traumatic scars in vitro and in vivo. Lasers Med Sci 28, 693–700 (2013). https://doi.org/10.1007/s10103-012-1127-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-012-1127-y