Abstract
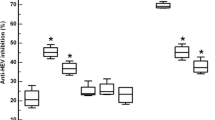
Hepatitis A virus (HAV) is a highly infectious agent that causes acute liver disease. The infection can trigger the production of antibodies against the structural and non-structural proteins of HAV. Nonetheless, vaccination with an HAV vaccine leads to the production of a primary antibody against the structural proteins. Because the non-structural proteins are only produced during active virus replication, there is no or very little antibody production against the non-structural proteins. However, the current commercial immunoassay cannot distinguish between antibodies produced during natural infection and those from vaccination against HAV. In our study, six immune-dominant epitopes from the non-structural proteins were designed, synthesized, linked together and cloned into pGEX-5X-1 plasmid. The recombinant protein was expressed in E. coli and purified by Ni2+-coated magnetic agarose beads. Then the purified recombinant protein was used as an ELISA antigen to detect antibodies for HAV non-structural proteins in serum samples. Seventy-seven attenuated and 89 inactivated vaccinated samples collected from our previous phase IV study of HAV vaccines were detected by peptide ELISA developed in this study. The mean OD450 value for the vaccination samples and acute infection samples were 0.529 (0.486 for the attenuated group and 0.567 for the inactivated group) and 1.187, respectively. According to the receiver operating characteristic (ROC) curve, the sensitivity and specificity of the peptide ELISA were 93.80% and 91.00%, respectively. This peptide ELISA was confirmed to discriminate vaccine-induced immunity from natural infection of HAV in a phase IV study with high sensitivity and specificity.


Similar content being viewed by others
References
Vaughan G, Goncalves Rossi LM, Forbi JC, de Paula VS, Purdy MA, Xia G, Khudyakov YE (2014) Hepatitis a virus: host interactions, molecular epidemiology and evolution. Infect Genet Evol 21:227–243
Nainan OV, Xia G, Vaughan G, Margolis HS (2006) Diagnosis of hepatitis a virus infection: a molecular approach. Clin Microbiol Rev 19:63–79
Hollinger FB, Emerson SU (2001) Hepatitis A virus. In: Knipe DM, Howley PM (eds) Fields Virology, fourth edn. Lippincott Williams & Wilkins, New York, pp 799–840
Cohen JI, Ticehurst JR, Purcell RH, Buckler-White A, Baroudy BM (1987) Complete nucleotide sequence of wild-type hepatitis a virus: comparison with different strains of hepatitis a virus and other picornaviruses. J Virol 61:50–59
Probst C, Jecht M, Gauss-Muller V (1999) Intrinsic signals for the assembly of hepatitis a virus particles. Role of structural proteins VP4 and 2A. J Biol Chem 274:4527–4531
Palmenberg A, Neubauer D, Skern T (2010) Genome organization andencoded proteins. In: Ehrenfeld E, Domingo E, Roos R (eds) The picornaviruses. ASM Press, Washington, DC, pp 3–17
Mao JS, Dong DX, Zhang HY, Chen NL, Zhang XY, Huang HY, Xie RY, Zhou TJ, Wan ZJ, Wang YZ et al (1989) Primary study of attenuated live hepatitis a vaccine (H2 strain) in humans. J Infect Dis 159:621–624
Wu JY, Liu Y, Chen JT, Xia M, Zhang XM (2012) Review of 10 years of marketing experience with Chinese domestic inactivated hepatitis a vaccine Healive(R). Hum Vaccin Immunother 8:1836–1844
Bradley DW, Maynard JE, Hindman SH, Hornbeck CL, Fields HA, McCaustland KA, Cook EH Jr (1977) Serodiagnosis of viral hepatitis a: detection of acute-phase immunoglobulin M anti-hepatitis a virus by radioimmunoassay. J Clin Microbiol 5:521–530
Shen YG, Gu XJ, Zhou JH (2008) Protective effect of inactivated hepatitis a vaccine against the outbreak of hepatitis a in an open rural community. World J Gastroenterol 14:2771–2775
Zhang LJ, Wang XJ, Bai JM et al (2009) An outbreak of hepatitis a in recently vaccinated students from ice snacks made from contaminated well water. Epidemiol Infect 137:428–433
Robertson BH, Jia XY, Tian H, Margolis HS, Summers DF, Ehrenfeld E (1992) Serological approaches to distinguish immune response to hepatitis a vaccine and natural infection. Vaccine 10(Suppl 1):S106–S109
Robertson BH, Jia XY, Tian H, Margolis HS, Summers DF, Ehrenfeld E (1993) Antibody response to nonstructural proteins of hepatitis a virus following infection. J Med Virol 40:76–82
Cowan KM, Graves JH (1966) A third antigenic component associated with foot-and-mouth disease infection. Virology 30:528–540
Robertson BH, Morgan DO, Moore DM, Grubman MJ, Card J, Fischer T, Weddell G, Dowbenko D, Yansura D (1983) Identification of amino acid and nucleotide sequence of the foot-and-mouth disease virus RNA polymerase. Virology 126:614–623
Neitzart E, Beck E, De Mello PA, Gomes I, Bergmann IE (1991) Expression of the apthovirus RNA polymerase gene in Escherichia coli and its use together with other bioengineered nonstructural antigens in detection of late persistentinfections. Virology 184:799–804
Ma F, Yang J, Kang G, Sun Q, Lu P, Zhao Y, Wang Z, Luo J, Wang Z (2016) Comparison of the safety and immunogenicity of live attenuated and inactivated hepatitis a vaccine in healthy Chinese children aged 18 months to 16 years: results from a randomized, parallel controlled, phase IV study. Clin Microbiol Infect 22:811.e819-811.e815
Khudyakov YE, Lopareva EN, Jue DL, Fang S, Spelbring J, Krawczynski K, Margolis HS, Fields HA (1999) Antigenic epitopes of the hepatitis a virus polyprotein. Virology 260:260–272
Liu J, Ye X, Jia J, Zhu R, Wang L, Chen C, Yang L, Wang Y, Wang W, Ye J, Li Y, Zhu H, Zhao Q, Cheng T, Xia N (2016) Serological evaluation of immunity to the varicella-zoster virus based on a novel competitive enzyme-linked immunosorbent assay. Sci Rep 6:20577
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Funding
This research was supported by the National “Twelfth Five Year” “major new drug discovery” technology major projects (grant no. 2012ZX09104–302), the Foundation of the CAMS Initiative for Innovative Medicine (CAMS-I2M) (grant no. 2016-I2M-1-19), and the Natural Science Foundation of Yunnan Province (2012FB188 and 2016FA029).
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All participants were informed of the study aims, and written informed consent was received from each participant before sample collection. The study protocol was approved by the Institutional Ethics Committee (Institute of Medical Biology, Chinese Academy of Medical Sciences, and Peking Union Medical College) and was in accordance with the Declaration of Helsinki for Human Research of 1974 (last modified in 2000).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Fig. S1
DNA sequence and the peptides of recombinant protein. The underlined sequences were endonuclease site sequences of BamH I(above) and EcoR I (below), respectively. (GIF 144 kb)
Fig. S2
(A) Bacterial colony PCR.Lane 1, E. coli BL21 cells without recombinant protein. Lane 2–7, E. coli BL21 cells successfully transformed with plasmids expressing recombinant protein. (B) Restriction enzyme digestion. Lane 1–2, empty vector. Lane3–4, recombinant vector. Target DNA was indicated by the arrow. (ZIP 232 kb)
Fig. S3
Lanes 1–3, E. coli cells transformed with empty vector. Lane 1, not induced; lane 2, induced for 4 h; lane 3, induced for 6 h; Lanes 4–6, E. coli cells transformed with recombinant plasmid. Lane 4, not induced; lane 5, induced for 4 h; lane 6: induced for 6 h. (GIF 116 kb)
Fig. S4
Purification of recombinant protein. Lane 1, E. coli cells transformed with empty vector induced 6 h; lane 2, total protein after sonication; lane 3, supernatant after centrifugation of inclusion bodies washed with 2 M urea. Lane 4, flow-through of NI2+ coated magnetic agarose beads; lane 5, eluate washed with 300 mM imidazole. Lane 6, eluate washed with 400 mM imidazole; lane 7, eluate washed with 500 mM imidazole. (GIF 83 kb)
Rights and permissions
About this article
Cite this article
Ye, C., Luo, J., Wang, X. et al. Development of a peptide ELISA to discriminate vaccine-induced immunity from natural infection of hepatitis A virus in a phase IV study. Eur J Clin Microbiol Infect Dis 36, 2165–2170 (2017). https://doi.org/10.1007/s10096-017-3040-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-3040-6