Abstract
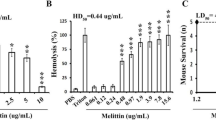
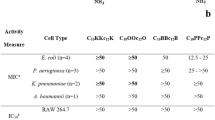
Pan-resistant Acinetobacter baumannii have prompted the search for therapeutic alternatives. We evaluate the efficacy of four cecropin A-melittin hybrid peptides (CA-M) in vivo. Toxicity was determined in mouse erythrocytes and in mice (lethal dose parameters were LD0, LD50, LD100). Protective dose 50 (PD50) was determined by inoculating groups of ten mice with the minimal lethal dose of A. baumannii (BMLD) and treating with doses of each CA-M from 0.5 mg/kg to LD0. The activity of CA-Ms against A. baumannii was assessed in a peritoneal sepsis model. Mice were sacrificed at 0 and 1, 3, 5, and 7-h post-treatment. Spleen and peritoneal fluid bacterial concentrations were measured. CA(1–8)M(1–18) was the less haemolytic on mouse erythrocytes. LD0 (mg/kg) was 32 for CA(1–8)M(1–18), CA(1–7)M(2–9), and Oct-CA(1–7)M(2–9), and 16 for CA(1–7)M(5–9). PD50 was not achieved with non-toxic doses (≤LD0). In the sepsis model, all CA-Ms were bacteriostatic in spleen, and decreased bacterial concentration (p < 0.05) in peritoneal fluid, at 1-h post-treatment; at later times, bacterial regrowth was observed in peritoneal fluid. CA-Ms showed local short-term efficacy in the peritoneal sepsis model caused by pan-resistant Acinetobacter baumannii.




Similar content being viewed by others
References
Rodriguez-Bano J, Cisneros JM, Fernandez-Cuenca F, Ribera A, Vila J, Pascual A, Martinez-Martinez L, Bou G, Pachon J (2004) Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 25:819–824
Vila J, Pachon J (2008) Therapeutic options for Acinetobacter baumannii infections. Expert Opin Pharmacother 9:587–599
Fournier PE, Richet H (2006) The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Infect Dis 42:692–699
Villegas MV, Hartstein AI (2003) Acinetobacter outbreaks, 1977–2000. Infect Control Hosp Epidemiol 24:284–295
Poirel L, Nordmann P (2006) Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology. Clin Microbiol Infect 12:826–836
Towner KJ, Levi K, Vlassiadi M (2008) Genetic diversity of carbapenem-resistant isolates of Acinetobacter baumannii in Europe. Clin Microbiol Infect 14:161–167
Valencia R, Arroyo LA, Conde M, Aldana JM, Torres MJ, Fernandez-Cuenca F, Garnacho-Montero J, Cisneros JM, Ortiz C, Pachon J, Aznar J (2009) Nosocomial outbreak of infection with pan-drug-resistant Acinetobacter baumannii in a tertiary care university hospital. Infect Control Hosp Epidemiol 30:257–263
Ganz T (2004) Defensins: antimicrobial peptides of vertebrates. C R Biol 327:539–549
Giuliani A, Nicoletto SF (2007) Antimicrobial peptides: an overview of a promising class of therapeutics. Cent Eur J Biol 2:33
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19:491–511
Khandelia H, Ipsen JH, Mouritsen OG (2008) The impact of peptides on lipid membranes. Biochim Biophys Acta 1778:1528–1536
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788:1687–1692
Rivas L, Andreu D (2003) Cecropin-melittin hybrid peptides as versatile templates in the development of membrane active antibiotics agents. In: Menestrina G, Dalla Serra M (eds) Pore-forming peptides and protein toxins. Harwood Academic Publishers, Reading, Berkshire, United Kingdom, pp 215–259
Giacometti A, Cirioni O, Ghiselli R, Mocchegiani F, D'Amato G, Del Prete MS, Orlando F, Kamysz W, Lukasiak J, Saba V, Scalise G (2003) Administration of protegrin peptide IB-367 to prevent endotoxin induced mortality in bile duct ligated rats. Gut 52:874–878
Ghiselli R, Giacometti A, Cirioni O, Orlando F, Mocchegiani F, Pacci AM, Scalise G, Saba V (2001) Therapeutic efficacy of the polymyxin-like peptide ranalexin in an experimental model of endotoxemia. J Surg Res 100:183–188
Giuliani A, Pirri G, Bozzi A, Di Giulio A, Aschi M, Rinaldi AC (2008) Antimicrobial peptides: natural templates for synthetic membrane-active compounds. Cell Mol Life Sci 65:2450–2460
Zelezetsky I, Tossi A (2006) Alpha-helical antimicrobial peptides—using a sequence template to guide structure-activity relationship studies. Biochim Biophys Acta 1758(9):1436–1449
Andreu D, Ubach J, Boman A, Wahlin B, Wade D, Merrifield RB, Boman HG (1992) Shortened cecropin A-melittin hybrids. Significant size reduction retains potent antibiotic activity. FEBS Lett 296(2):190–194
Boman HC, Boman IA, Andreu D, Li ZQ, Merrifield RB, Schlenstedt G, Zimmermann R (1989) Chemical synthesis and enzymic processing of precursor forms of cecropins A and B. J Biol Chem 264(10):5852–5860
Cavallarin L, Andreu D, San Segundo B (1998) Cecropin A-derived peptides are potent inhibitors of fungal plant pathogens. Mol Plant Microbe Interact 11(3):218–227
Chicharro C, Granata C, Lozano R, Andreu D, Rivas L (2001) N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1–7)M(2–9), a cecropin-melittin hybrid peptide. Antimicrob Agents Chemother 45(9):2441–2449
Friedrich C, Scott MG, Karunaratne N, Yan H, Hancock RE (1999) Salt-resistant alpha-helical cationic antimicrobial peptides. Antimicrob Agents Chemother 43(7):1542–1548
Rodriguez-Hernandez MJ, Saugar J, Docobo-Perez F, de la Torre BG, Pachon-Ibanez ME, Garcia-Curiel A, Fernandez-Cuenca F, Andreu D, Rivas L, Pachon J (2006) Studies on the antimicrobial activity of cecropin A-melittin hybrid peptides in colistin-resistant clinical isolates of Acinetobacter baumannii. J Antimicrob Chemother 58(1):95–100
Saugar JM, Rodriguez-Hernandez MJ, de la Torre BG, Pachon-Ibanez ME, Fernandez-Reyes M, Andreu D, Pachon J, Rivas L (2006) Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: molecular basis for the differential mechanisms of action. Antimicrob Agents Chemother 50(4):1251–1256
C57BL/6 Datasheet. Harland. http://www.harlan.com/research_models_and_services/research_models_by_product_type/inbred_mice/c57bl6j_inbred_mice.hl. Accessed 13 December 2010.
BALBc Datasheet. Harland. http://www.harlan.com/research_models_and_services/research_models_by_product_type/inbred_mice/balbc.hl. Accessed 13 December 2010.
Solanas C, de la Torre BG, Fernandez-Reyes M, Santiveri CM, Jimenez MA, Rivas L, Jimenez AI, Andreu D, Cativiela C (2009) Therapeutic index of gramicidin S is strongly modulated by D-phenylalanine analogues at the beta-turn. J Med Chem 52(3):664–674
O’Reilly T, Cleeland R, Squires EL (1996) Evaluation of antimicrobials in experimental animal infections. In: Lorian V (ed) Antibiotics in laboratory medicine. Williams and Wilkins, MD, USA, pp 604–765
Pachon J, Vila J (2009) Treatment of multiresistant Acinetobacter baumannii infections. Curr Opin Investig Drugs 10(2):150–156
Pereira HA (2006) Novel therapies based on cationic antimicrobial peptides. Curr Pharm Biotechnol 7(4):229–234
Zhang L, Falla TJ (2006) Antimicrobial peptides: therapeutic potential. Expert Opin Pharmacother 7(6):653–663
Braunstein A, Papo N, Shai Y (2004) In vitro activity and potency of an intravenously injected antimicrobial peptide and its DL amino acid analog in mice infected with bacteria. Antimicrob Agents Chemother 48(8):3127–3129
Giacometti A, Cirioni O, Kamysz W, D'Amato G, Silvestri C, Del Prete MS, Lukasiak J, Scalise G (2003) Comparative activities of cecropin A, melittin, and cecropin A-melittin peptide CA(1–7)M(2–9)NH2 against multidrug-resistant nosocomial isolates of Acinetobacter baumannii. Peptides 24(9):1315–1318
Saugar JM, Alarcon T, Lopez-Hernandez S, Lopez-Brea M, Andreu D, Rivas L (2002) Activities of polymyxin B and cecropin A-, melittin peptide CA(1–8)M(1–18) against a multiresistant strain of Acinetobacter baumannii. Antimicrob Agents Chemother 46(3):875–878
Avrahami D, Shai Y (2003) Bestowing antifungal and antibacterial activities by lipophilic acid conjugation to D, L-amino acid-containing antimicrobial peptides: a plausible mode of action. Biochemistry 42(50):14946–14956
Gough M, Hancock RE, Kelly NM (1996) Antiendotoxin activity of cationic peptide antimicrobial agents. Infect Immun 64(12):4922–4927
Zhang L, Falla T, Wu M, Fidai S, Burian J, Kay W, Hancock RE (1998) Determinants of recombinant production of antimicrobial cationic peptides and creation of peptide variants in bacteria. Biochem Biophys Res Commun 247(3):674–680
Seebach D, Beck AK, Bierbaum DJ (2004) The world of beta- and gamma-peptides comprised of homologated proteinogenic amino acids and other components. Chem Biodivers 1(8):1111–1239
Zhang L, Benz R, Hancock RE (1999) Influence of proline residues on the antibacterial and synergistic activities of alpha-helical peptides. Biochemistry 38(25):8102–8111
Piers KL, Brown MH, Hancock RE (1994) Improvement of outer membrane-permeabilizing and lipopolysaccharide-binding activities of an antimicrobial cationic peptide by C-terminal modification. Antimicrob Agents Chemother 38(10):2311–2316
Acknowledgement
This work was supported by the Spanish Ministry of Science and Innovation (grant PET2006-0139 to D.A. and L.R.), and by the Spanish Ministry of Health (Fondo de Investigaciones Sanitarias-FEDER, grants PI061125 and PI040827 to L.R., PI040885 to D.A., PI041854 to J.A.B. and PI040624 to J.P.) Additional funding from the regional governments of Madrid (S-BIO-0260/2006 to L.R.) and Catalonia (SGR2005-00494 to D.A.) is acknowledged. L.R. belongs to the COMBACT Network (BIO260) (Comunidad Autónoma de Madrid). J.P. belongs to the Spanish Network for Research in Infectious Diseases (REIPI RD06/0008), the Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III-FEDER. CIBERES is an initiative from Instituto de Salud Carlos III.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Rojas, R., Docobo-Pérez, F., Pachón-Ibáñez, M.E. et al. Efficacy of cecropin A-melittin peptides on a sepsis model of infection by pan-resistant Acinetobacter baumannii . Eur J Clin Microbiol Infect Dis 30, 1391–1398 (2011). https://doi.org/10.1007/s10096-011-1233-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-011-1233-y