Abstract
Introduction
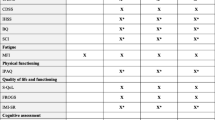
Fatigue is a prominent symptom in post-COVID condition (PCC) sequelae, termed “long COVID.” Herein, we aim to ascertain the effect of fatigue on psychosocial function in persons living with PCC.
Methods
This post hoc analysis evaluated the effects of vortioxetine on measures of fatigue as assessed by the Fatigue Severity Scale (FSS) in psychosocial function as measured by the Sheehan Disability Scale (SDS) in persons with PCC. We also evaluated the change in FSS on psychosocial functioning as measured by the Sheehan Disability Scale (SDS). This post hoc analysis obtained data from a recently published placebo-controlled study evaluating vortioxetine’s effect on objective cognitive functions in persons living with PCC.
Results
One hundred forty-four participants meeting World Health Organization (WHO) criteria for PCC were included in this analysis. At the end of 8 weeks of vortioxetine treatment, significant improvement of all domains was observed for psychosocial functioning. There was a significant between-group difference at treatment endpoint in the family, social, and work SDS subcategories (p < 0.001). There was a statistically significant interaction effect between the treatment condition time point and FSS effect on the SDS social (χ2 = 10.640, p = 0.014) and work (χ2 = 9.342, p = 0.025) categories but a statistically insignificant effect on the family categories ((χ2 = 5.201, p = 0.158)).
Discussion
This post hoc analysis suggests that vortioxetine treatment significantly improves psychosocial function in persons with PCC. Our results also indicate that the improvement in psychosocial function was significantly mediated by improvement in measures of fatigue. Our results provide empirical support for recommendations to identify therapeutics for fatigue in persons living with PCC with a broader aim to improve psychosocial function in this common and severely impaired population.
Similar content being viewed by others
References
Davis HE, Assaf GS, McCorkell L et al (2021) Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38:101019. https://doi.org/10.1016/j.eclinm.2021.101019
Walker S, Goodfellow H, Pookarnjanamorakot P et al (2023) Impact of fatigue as the primary determinant of functional limitations among patients with post-COVID-19 syndrome: a cross-sectional observational study. BMJ Open 13(6):e069217. https://doi.org/10.1136/bmjopen-2022-069217
Bonilla H, Peluso MJ, Rodgers K et al (2023) Therapeutic trials for long COVID-19: a call to action from the interventions taskforce of the RECOVER initiative. Front Immunol 14:1129459. https://doi.org/10.3389/fimmu.2023.1129459
Murga I, Aranburu L, Gargiulo PA, Gómez Esteban JC, Lafuente JV (2021) Clinical heterogeneity in ME/CFS. A way to understand long-COVID19 fatigue. Front Psychiatry 12. https://www.frontiersin.org/articles/10.3389/fpsyt.2021.735784. Accessed June 19, 2023
Rudroff T, Fietsam AC, Deters JR, Bryant AD, Kamholz J (2020) Post-COVID-19 fatigue: potential contributing factors. Brain Sci 10(12):1012. https://doi.org/10.3390/brainsci10121012
Taylor AK, Kingstone T, Briggs TA et al (2021) “Reluctant pioneer”: a qualitative study of doctors’ experiences as patients with long COVID. Health Expect Int J Public Particip Health Care Health Policy 24(3):833–842. https://doi.org/10.1111/hex.13223
Kedor C, Freitag H, Meyer-Arndt L, et al. Chronic COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) following the first pandemic wave in Germany–a first analysis of a prospective observational study. Published online February 8, 2021:2021.02.06.21249256. https://doi.org/10.1101/2021.02.06.21249256
Twomey R, DeMars J, Franklin K, Culos-Reed SN, Weatherald J, Wrightson JG (2022) Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys Ther 102(4):pzac005. https://doi.org/10.1093/ptj/pzac005
Vink M, Vink-Niese A (2020) Could cognitive behavioural therapy be an effective treatment for long COVID and post COVID-19 fatigue syndrome? Lessons from the Qure study for Q-fever fatigue syndrome. Healthc Basel Switz 8(4):552. https://doi.org/10.3390/healthcare8040552
D’Agostino A, English CD, Rey JA (2015) Vortioxetine (Brintellix): a new serotonergic antidepressant. Pharm Ther 40(1):36–40
Santos García D, Alonso Losada MG, Cimas Hernando I et al (2022) Vortioxetine improves depressive symptoms and cognition in Parkinson’s disease patients with major depression: an open-label prospective study. Brain Sci 12(11):1466. https://doi.org/10.3390/brainsci12111466
McIntyre RS, Phan L, Kwan ATH, et al. Vortioxetine for the treatment of post-COVID-19 condition: a randomized controlled trial. Brain. Published online November 4, 2023:awad377. https://doi.org/10.1093/brain/awad377
Cao B, Park C, Rosenblat JD et al (2019) Changes in sleep predict changes in depressive symptoms in depressed subjects receiving vortioxetine: an open-label clinical trial. J Psychopharmacol Oxf Engl 33(11):1388–1394. https://doi.org/10.1177/0339881119874485
Liguori C, Ferini-Strambi L, Izzi F et al (2019) Preliminary evidence that vortioxetine may improve sleep quality in depressed patients with insomnia: a retrospective questionnaire analysis. Br J Clin Pharmacol 85(1):240–244. https://doi.org/10.1111/bcp.13772
Leigh E, Clark DM (2018) Understanding social anxiety disorder in adolescents and improving treatment outcomes: applying the cognitive model of Clark and Wells (1995). Clin Child Fam Psychol Rev 21(3):388–414. https://doi.org/10.1007/s10567-018-0258-5
Navaneetham P, Kanth B (2022) Effects of personal relationships on physical and mental health among young adults- a scoping review. Open Psychol J 15(1). https://doi.org/10.2174/18743501-v15-e2208180
Wu B (2020) Social isolation and loneliness among older adults in the context of COVID-19: a global challenge. Glob Health Res Policy 5(1):27. https://doi.org/10.1186/s41256-020-00154-3
Dawson D, Ian Noy Y, Härmä M, Åkerstedt T, Belenky G (2011) Modelling fatigue and the use of fatigue models in work settings. Accid Anal Prev 43(2):549–564. https://doi.org/10.1016/j.aap.2009.12.030
Majer M, Welberg LAM, Capuron L, Miller AH, Pagnoni G, Reeves WC (2008) Neuropsychological performance in persons with chronic fatigue syndrome: results from a population-based study. Psychosom Med 70(7):829–836. https://doi.org/10.1097/PSY.0b013e31817b9793
Trecroci A, Boccolini G, Duca M, Formenti D, Alberti G (2020) Mental fatigue impairs physical activity, technical and decision-making performance during small-sided games. PLoS ONE 15(9):e0238461. https://doi.org/10.1371/journal.pone.0238461
Aoun Sebaiti M, Hainselin M, Gounden Y et al (2022) Systematic review and meta-analysis of cognitive impairment in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Sci Rep 12(1):2157. https://doi.org/10.1038/s41598-021-04764-w
Shockey TM (2017) Short sleep duration by occupation group — 29 states, 2013–2014. MMWR Morb Mortal Wkly Rep 66. https://doi.org/10.15585/mmwr.mm6608a2
Caldwell JA, Caldwell JL, Thompson LA, Lieberman HR (2019) Fatigue and its management in the workplace. Neurosci Biobehav Rev 96:272–289. https://doi.org/10.1016/j.neubiorev.2018.10.024
Brown R, Wey H, Foland K (2018) The relationship among change fatigue, resilience, and job satisfaction of hospital staff nurses. J Nurs Scholarsh 50(3):306–313. https://doi.org/10.1111/jnu.12373
Fan J, Smith AP (2017) The impact of workload and fatigue on performance. In: Longo L, Leva MC (eds) Human mental workload: models and applications. Communications in computer and information science. Springer International Publishing, pp 90–105. https://doi.org/10.1007/978-3-319-61061-0_6
Gil-Sanchez A, Canudes M, Valcheva P et al (2024) Effects of vortioxetine on cognition and fatigue in patients with multiple sclerosis and depression: a case series study. CNS Neurol Disord Drug Targets 23(3):395–401. https://doi.org/10.2174/1871527323366230321093133
Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M (2023) Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry 28(2):564–578. https://doi.org/10.1038/s41380-022-01836-9
Talmon M, Rossi S, Pastore A, Cattaneo CI, Brunelleschi S, Fresu LG (2018) Vortioxetine exerts anti-inflammatory and immunomodulatory effects on human monocytes/macrophages. Br J Pharmacol 175(1):113–124. https://doi.org/10.1111/bph.14074
Yoo EH, Choi ES, Cho SH, Do JH, Lee SJ, Kim JH (2018) Comparison of fatigue severity and quality of life between unexplained fatigue patients and explained fatigue patients. Korean J Fam Med 39(3):180–184. https://doi.org/10.4082/kjfm.2018.39.3.180
Daumas L, Corbel C, Zory R et al (2022) Associations, overlaps and dissociations between apathy and fatigue. Sci Rep 12(1):7387. https://doi.org/10.1038/s41598-022-11071-5
Slimani M, Znazen H, Bragazzi NL, Zguira MS, Tod D (2018) The effect of mental fatigue on cognitive and aerobic performance in adolescent active endurance athletes: insights from a randomized counterbalanced, cross-over trial. J Clin Med 7(12):510. https://doi.org/10.3390/jcm7120510
Di Nicola M, Pepe M, Montanari S et al (2023) Vortioxetine improves physical and cognitive symptoms in patients with post-COVID-19 major depressive episodes. Eur Neuropsychopharmacol 70:21–28. https://doi.org/10.1016/j.euroneuro.2023.02.006
Baldwin DS, Florea I, Jacobsen PL, Zhong W, Nomikos GG (2016) A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord 206:140–150. https://doi.org/10.1016/j.jad.2016.07.015
Knight MJ, Baune BT (2018) Cognitive dysfunction in major depressive disorder. Curr Opin Psychiatry 31(1):33–31. https://doi.org/10.1097/YCO.0000000000000378
Marin RS, Wilkosz PA (2005) Disorders of diminished motivation. J Head Trauma Rehabil 20(4):377
Pozzi C, Sarti R, Levi R et al (2023) Association between duration of SARS-CoV-2 positivity and long COVID. Clin Infect Dis 77(11):1531–1533. https://doi.org/10.1093/cid/ciad434
Sudre CH, Murray B, Varsavsky T et al (2021) Attributes and predictors of long COVID. Nat Med 27(4):633–631. https://doi.org/10.1038/s41591-021-01292-y
Westerlind E, Palstam A, Sunnerhagen KS, Persson HC (2021) Patterns and predictors of sick leave after Covid-19 and long Covid in a national Swedish cohort. BMC Public Health 21(1):1023. https://doi.org/10.1186/s12889-021-11013-2
Dreyer N, Petruski-Ivleva N, Albert L et al (2021) Identification of a vulnerable group for post-acute sequelae of SARS-CoV-2 (PASC): people with autoimmune diseases recover more slowly from COVID-19. Int J Gen Med 14:3941–3949. https://doi.org/10.2147/IJGM.S313486
Aminian A, Bena J, Pantalone KM, Burguera B (2021) Association of obesity with postacute sequelae of COVID-19. Diabetes Obes Metab 23(9):2183–2188. https://doi.org/10.1111/dom.14454
Barrea L, Grant WB, Frias-Toral E et al (2022) Dietary recommendations for post-COVID-19 syndrome. Nutrients 14(6):1305. https://doi.org/10.3390/nu14061305
Jimeno-Almazán A, Pallarés JG, Buendía-Romero Á et al (2021) Post-COVID-19 syndrome and the potential benefits of exercise. Int J Environ Res Public Health 18(10):5329. https://doi.org/10.3390/ijerph18105329
Vu T, McGill SC (2021) An overview of post–COVID-19 condition (long COVID). Can J Health Technol 1(9). https://doi.org/10.51731/cjht.2021.160
Huang L, Yao Q, Gu X et al (2021) 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet Lond Engl 398(10302):747–758. https://doi.org/10.1016/S0140-6736(21)01755-4
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval and consent to participate
This study was conducted in accordance with the principles of Good Clinical Practice (ICH, 1996) and the Declaration of Helsinki (WMA, 2008). A local research ethics board (REB) approved the trial design and all eligible participants provided written informed consent before enrollment. This study was registered on ClinicalTrials.gov (NCT05047952).
Conflict of interest
Dr. Roger S. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC) and the Milken Institute and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Neurawell, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, Abbvie and Atai Life Sciences. Dr. S. Roger McIntyre is a CEO of Braxia Scientific Corp.
Kayla M. Teopiz has received fees from Braxia Scientific Corp.
Dr. Joshua D. Rosenblat has received research grant support from the Canadian Institute of Health Research (CIHR), Physician Services Inc. (PSI) Foundation, Labatt Brain Health Network, Brain and Cognition Discovery Foundation (BCDF), Canadian Cancer Society, Canadian Psychiatric Association, Academic Scholars Award, American Psychiatric Association, American Society of Psychopharmacology, University of Toronto, University Health Network Centre for Mental Health, Joseph M. West Family Memorial Fund, and Timeposters Fellowship and industry funding for speaker/consultation/research fees from iGan, Boehringer Ingelheim, Janssen, Allergan, Lundbeck, Sunovion, and COMPASS.
Dr. Rodrigo B. Mansur has received research grant support from the Canadian Institute of Health Research; Physicians’ Services Incorporated Foundation; the Baszucki Brain Research Fund; and the Academic Scholar Awards, Department of Psychiatry, University of Toronto.
All other authors (Sebastian Badulescu, Gia Han Le, Sabrina Wong, Angela T.H. Kwan, Ziji Guo, Lee Phan, and Mehala Subramaniapillai) have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Badulescu, S., Le, G.H., Wong, S. et al. Impact of vortioxetine on psychosocial functioning moderated by symptoms of fatigue in post-COVID-19 condition: a secondary analysis. Neurol Sci 45, 1335–1342 (2024). https://doi.org/10.1007/s10072-024-07377-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-024-07377-z