Abstract
Background
Many studies have reported reduced brain white matter fractional anisotropy (FA) and increased mean diffusivity (MD) on diffusion tensor imaging (DTI) of people with HIV (PWH). Few, however, have linked individual blood inflammatory markers with white matter tract-specific FA and MD.
Methods
PWH 50 years old or older from New York, NY, USA, were invited to a cross-sectional study. Demographic data, blood samples, and brain DTI were obtained. Least absolute shrinkage and selection operator (LASSO) regression was used to examine associations between biomarkers and white matter tract-specific FA and MD. All models included age, sex, race, ethnicity, diabetes, hypertension, smoking, and viral load as control variables.
Results
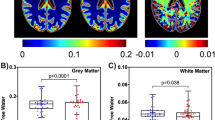
Seventy-two cases were analyzed. Mean age was 60 ± 6 years, 47% were women, 21% were Hispanic, and 78% were black. All had asymptomatic HIV infection and were on antiretroviral therapy. Eighty-nine percent had CD4 count >200 cell/mm3 and 78% were virally suppressed. Vascular endothelial growth factor (VEGF) and macrophage inflammatory proteins (MIP) 1β and 1α were consistently associated with lower FA and higher MD across white matter tracts.
Conclusions
Elevated serum VEGF, MIP-1α, and MIP-1β were associated with altered white matter microstructure. These blood biomarkers may help predict HIV-associated white matter damage.
Similar content being viewed by others
References
Marcus JL, Leyden WA, Alexeeff SE et al (2020) Comparison of overall and comorbidity-free life expectancy between insured adults with and without HIV infection, 2000–2016. JAMA Netw Open 3:e207954-e
Sacktor N (2018) Changing clinical phenotypes of HIV-associated neurocognitive disorders. J Neurovirol 24:141–5
Saloner R, Cysique LA (2017) HIV-associated neurocognitive disorders: a global perspective. J Int Neuropsychol Soc 23:860–9
Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E (2007) Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol 28:226–35
Rose SE, McMahon KL, Janke AL et al (2006) Diffusion indices on magnetic resonance imaging and neuropsychological performance in amnestic mild cognitive impairment. J Neurol Neurosurg Psychiatry 77:1122–8
Stebbins GT, Smith CA, Bartt RE et al (2007) HIV-associated alterations in normal-appearing white matter: a voxel-wise diffusion tensor imaging study. J Acquir Immune Defic Syndr 46:564–73
Wright P, Heaps J, Shimony JS, Thomas JB, Ances BMJA (2012) The effects of HIV and combination antiretroviral therapy on white matter integrity. AIDS 26:1501
Gongvatana A, Cohen RA, Correia S et al (2011) Clinical contributors to cerebral white matter integrity in HIV-infected individuals. J Neurovirol 17:477
Raja F, Sherriff F, Morris C, Bridges L, Esiri MM (1997) Cerebral white matter damage in HIV infection demonstrated using β-amyloid precursor protein immunoreactivity. Acta Neuropathol 93:184–9
McWhinney SR, Tremblay A, Chevalier TM, Lim VK, Newman AJ (2016) Using CForest to analyze diffusion tensor imaging data: a study of white matter integrity in healthy aging. Brain Connect 6:747–58
Sexton CE, Walhovd KB, Storsve AB et al (2014) Accelerated changes in white matter microstructure during aging: a longitudinal diffusion tensor imaging study. J Neurosci 34:15425–36
Chang K, Premeaux TA, Cobigo Y et al (2020) Plasma inflammatory biomarkers link to diffusion tensor imaging metrics in virally suppressed HIV-infected individuals. AIDS 34:203
Cohen RA, de la Monte S, Gongvatana A et al (2011) Plasma cytokine concentrations associated with HIV/hepatitis C coinfection are related to attention, executive and psychomotor functioning. J Neuroimmunol 233:204–10
Falasca K, Reale M, Ucciferri C et al (2017) Cytokines, hepatic fibrosis, and antiretroviral therapy role in neurocognitive disorders HIV related. AIDS Res Hum Retroviruses 33:246–253
Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O (2010) Soluble TNF receptors are associated with Aβ metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging 31:1877–1884
Yuan L, Liu A, Qiao L et al (2015) The relationship of CSF and plasma cytokine levels in HIV infected patients with neurocognitive impairment. BioMed research Int 2015:1–5
Gutierrez J, Porras TN, Yoo-Jeong M et al (2021) Cerebrovascular contributions to neurocognitive disorders in people living With HIV. J Acquir Immune Defic Syndr 88:79–85
Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H (1983) Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry 140(6):734–739
Tibshirani R (1996) Regression shrinkage and selection via the lasso. Journal of the Royal 58:267–88
Hastie T, Tibshirani R, Friedman JH (2009) The elements of statistical learning: data mining, inference, and prediction, vol 2. Springer, New York, pp 1–758
Corrêa DG, Zimmermann N, Doring TM et al (2015) Diffusion tensor MR imaging of white matter integrity in HIV-positive patients with planning deficit. Neuroradiology 57:475–82
Zhu T, Zhong J, Hu R et al (2013) Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. J Neurovirol 19:10–23
Ascherl G, Hohenadl C, Schatz O et al (1999) Infection with human immunodeficiency virus-1 increases expression of vascular endothelial cell growth factor in T cells: implications for acquired immunodeficiency syndrome-associated vasculopathy. Blood 93:4232–41
Heidenreich F, Arendt G, Jander S, Jablonowski H, Stoll G (1994) Serum and cerebrospinal fluid levels of soluble intercellular adhesion molecule 1 (sICAM-1) in patients with HIV-1 associated neurological diseases. Journal of Neuroimmunology 52:117–26
Glass JD, Fedor H, Wesselingh SL, McArthur JC (1995) Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol 38(5):755–762
Sporer B, Koedel U, Paul R et al (2004) Vascular endothelial growth factor (VEGF) is increased in serum, but not in cerebrospinal fluid in HIV associated CNS diseases. Journal of Neurology 75:298–300
Cocchi F, DeVico AL, Yarchoan R et al (2000) Higher macrophage inflammatory protein (MIP)-1α and MIP-1β levels from CD8+ T cells are associated with asymptomatic HIV-1 infection. Proc Natl Acad Sci U S A 97:13812–7
Levine AJ, Soontornniyomkij V, Achim CL et al (2016) Multilevel analysis of neuropathogenesis of neurocognitive impairment in HIV. J Neurovirol 22:431–41
Wagner L, Yang OO, Garcia-Zepeda EA et al (1998) β-chemokines are released from HIV-1-specific cytolytic T-cell granules complexed to proteoglycans. Nature 391:908–11
Burdo TH, Weiffenbach A, Woods SP, Letendre S, Ellis RJ, Williams KCJA (2013) Elevated sCD163 in plasma but not cerebrospinal fluid is a marker of neurocognitive impairment in HIV infection. AIDS 27(9):1387–1395
Imp BM, Rubin LH, Tien PC et al (2017) Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis 215:114
Reiber H (2001) Dynamics of brain-derived proteins in cerebrospinal fluid. Clin Chim Acta 310:173–186
Reiber H (2003) Proteins in cerebrospinal fluid and blood: barriers, CSF flow rate and source-related dynamics. Restor Neurol Neurosci 21:79–96
Reiber H, Padilla-Docal B, Jensenius JC, Dorta-Contreras AJ (2012) Mannan-binding lectin in cerebrospinal fluid: a leptomeningeal protein. Fluids and Barriers of the CNS 9:1–7
de Almeida SM, Rotta I, Ribeiro CE et al (2016) Blood-CSF barrier and compartmentalization of CNS cellular immune response in HIV infection. J Neuroimmunol 301:41–48
Kothur K, Wienholt L, Brilot F, Dale RCJC (2016) CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: a systematic review. Cytokine 77:227–37
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
None.
Conflict of interest
None.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spagnolo-Allende, A., Schnall, R., Liu, M. et al. Serum inflammation markers associated with altered brain white matter microstructure in people with HIV on antiretroviral treatment. Neurol Sci 44, 2159–2166 (2023). https://doi.org/10.1007/s10072-023-06613-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06613-2