Abstract
Background
Natalizumab (NAT) has a strong impact on disease activity of aggressive pediatric multiple sclerosis (MS), with no difference in safety profile compared to adult MS. However, available data are limited by short follow-up.
Our aim was to report long-term follow-up data (up to 11 years) of a large Italian pediatric MS cohort treated with NAT.
Materials and methods
We retrospectively collected data of pediatric MS patients treated with NAT included in a previous study and prospectively followed in Italian MS centers. We compared disease activity pre, during, and post-NAT and we performed survival analyses of time to evidence of disease activity (EDA) during NAT, time to reach EDA post-NAT, and time to NAT discontinuation.
Results
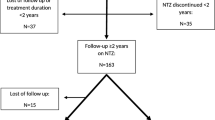
Ninety-two patients were included from 19 MS centers in Italy. At NAT initiation, cohort’s characteristics were as follows: 55 females; 14.7 ± 2.4 (mean ± SD) years of age; 34 naïve to disease modifying therapies; 1-year pre-NAT annualized relapse rate (ARR): 2.2 ± 1.2; EDSS (median [IQR]): 2.5 [2.0–3.0]; gadolinium-enhancing lesions: 2 [1–5]; 41 JCV positives. During NAT treatment (61.9 ± 35.2 mean infusions), ARR lowered to 0.08 ± 0.23 (p < 0.001), EDSS score to 1.5 [1.0–2.5] at last infusion (p < 0.001), and 51% patients had EDA (21% after 6 months of rebaseline). No serious adverse events were reported. Forty-nine patients discontinued NAT, mainly due to PML concern; the majority (29/49) had disease reactivation in the subsequent 12 months, of which three with a clinical rebound.
Conclusion
NAT treatment maintains its high efficacy for a long time in pediatric MS patients, with no new safety issues.





Similar content being viewed by others
References
Gorman MP, Healy BC, Polgar-Turcsanyi M, Chitnis T (2009) Increased relapse rate in pediatric-onset compared with adult-onset multiple sclerosis. Arch Neurol 66(1):54–59. https://doi.org/10.1001/archneurol.2008.505
Benson LA, Healy BC, Gorman MP et al (2014) Elevated relapse rates in pediatric compared to adult MS persist for at least 6 years. Mult Scler Relat Disord 3(2):186–193. https://doi.org/10.1016/j.msard.2013.06.004
Langille MM, Islam T, Burnett M, Amezcua L (2016) Clinical characteristics of pediatric-onset and adult-onset multiple sclerosis in Hispanic Americans. J Child Neurol 31(8):1068–1073. https://doi.org/10.1177/0883073816638754
Harding KE, Liang K, Cossburn MD et al (2013) Long-term outcome of paediatric-onset multiple sclerosis: a population-based study. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2012-303996
Amato MP, Krupp LB, Charvet LE, Penner I, Till C (2016) Pediatric multiple sclerosis: cognition and mood. Neurology 87(9 Suppl 2):S82–S87. https://doi.org/10.1212/WNL.0000000000002883
Baruch NF, O’Donnell EH, Glanz BI et al (2016) Cognitive and patient-reported outcomes in adults with pediatric-onset multiple sclerosis. Mult Scler 22(3):354–361. https://doi.org/10.1177/1352458515588781
McKay KA, Hillert J, Manouchehrinia A (2019) Long-term disability progression of pediatric-onset multiple sclerosis. Neurology. https://doi.org/10.1212/WNL.0000000000007647
Baroncini D, Simone M, Iaffaldano P et al (2021) Risk of persistent disability in patients with pediatric-onset multiple sclerosis. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2021.1008
Renoux C, Vukusic S, Mikaeloff Y et al (2007) Natural history of multiple sclerosis with childhood onset. N Engl J Med 356(25):2603–2613. https://doi.org/10.1056/NEJMoa067597
Gazzetta Ufficiale della Repubblica Italiana, serie generale n.292 del 16/12/2006.
Gazzetta Ufficiale della Repubblica Italiana, serie generale n.214 del 15–09–2014, page 26.
Ghezzi A, Moiola L, Pozzilli C et al (2015) Natalizumab in the pediatric MS population: results of the Italian registry. BMC Neurol 15:174. https://doi.org/10.1186/s12883-015-0433-y
Arnal-Garcia C, García-Montero MR, Málaga I et al (2013) Natalizumab use in pediatric patients with relapsing-remitting multiple sclerosis. Eur J Paediatr Neurol. https://doi.org/10.1016/j.ejpn.2012.09.004
Huppke P, Huppke B, Ellenberger D et al (2017) Therapy of highly active pediatric multiple sclerosis. Mult Scler. 25:72–80. https://doi.org/10.1177/1352458517732843
Kornek B, Aboul-Enein F, Rostasy K et al (2013) Natalizumab therapy for highly active pediatric multiple sclerosis. JAMA Neurol 70(4):469–475. https://doi.org/10.1001/jamaneurol.2013.923
Palavra F, Figueiroa S, Correia AS et al (2021) TyPed study: Natalizumab for the treatment of pediatric-onset multiple sclerosis in Portugal. Mult Scler Relat Disord. https://doi.org/10.1016/j.msard.2021.102865
Menascu S, Fattal-Valevski A, Vaknin-Dembinsky A et al (2022) Effect of natalizumab treatment on the rate of no evidence of disease activity in young adults with multiple sclerosis in relation to pubertal stage. J Neurol Sci. https://doi.org/10.1016/j.jns.2021.120074
Alroughani R, Ahmed SF, Behbehani R, Al-Hashel J (2017) The use of natalizumab in pediatric patients with active relapsing multiple sclerosis: a prospective study. Pediatr Neurol. https://doi.org/10.1016/j.pediatrneurol.2017.01.017
Margoni M, Rinaldi F, Riccardi A, Franciotta S, Perini P, Gallo P (2020) No evidence of disease activity including cognition (NEDA-3 plus) in naïve pediatric multiple sclerosis patients treated with natalizumab. J Neurol. https://doi.org/10.1007/s00415-019-09554-z
Ryerson LZ, Foley J, Chang I et al (2019) Risk of natalizumab-associated PML in patients with MS is reduced with extended interval dosing. Neurology. https://doi.org/10.1212/WNL.0000000000008243
Prosperini L, Scarpazza C, Imberti L, Cordioli C, De Rossi N, Capra R (2017) Age as a risk factor for early onset of natalizumab-related progressive multifocal leukoencephalopathy. J Neurovirol. https://doi.org/10.1007/s13365-017-0561-9
Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S (2019) Post-natalizumab disease reactivation in multiple sclerosis: systematic review and meta-analysis. Ther Adv Neurol Disord. https://doi.org/10.1177/1756286419837809
MS Study Group of the Italian Neurological Society: Maria Trojano (Bari), Diego Centonze (Pozzilli), Ruggero Capra (Montichiari), Marco Capobianco (Orbassano), Alice Laroni (Genova), Antonio Uccelli (Genova), Antonio Gallo (Napoli), Francesco Patti (Catania), Maura Chiara Danni (Ancona), Claudio Gasperini (Roma), Gabriella Coniglio (Matera)
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Disclosures
Authors
Damiano Baroncini received travel grants for participation at national/international congresses and compensations for consulting/speaking/scientific publications activities from Sanofi Genzyme, Teva, Merck, Biogen, Roche, Almirall, and Novartis.
Angelo Ghezzi received honoraria for speaking from Almirall, Biogen Idec, Merck Serono, Novartis, Genzyme and Sanofi-Aventis and for consultancy from Merck Serono, Biogen Idec, Teva, F Hoffmann-La Roche and Novartis.
Clara Guaschino has served on scientific advisory boards and received support for travel and congress attendance from Biogen, Novartis, Almirall, Sanofi-Genzyme, Merck-Serono.
Lucia Moiola has received honoraria for speaking and for partecipating to advisory board from: Biogen-Idec; Merck; Sanofi-Genzyme; Novartis; Celgene; Roche.
Massimo Filippi is Editor-in-Chief of the Journal of Neurology and Associate Editor of Human Brain Mapping, Neurological Sciences, and Radiology; received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, MerckSerono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA).
Antonio Ianniello reports no disclosures.
Carlo Pozzilli received consulting and lecture fees from Sanofi-Aventis, Biogen Idec, Bayer Schering, Merck Serono, and Novartis. He also received research funding from Novartis, Sanofi-Aventis, Merck Serono, and Bayer Schering.
Roberta Lanzillo has received honoraria from Merck, Novartis, Roche, Sanofi-Genzyme and Teva.
Vincenzo Brescia-Morra has received research grants from the Italian MS Society and Roche and honoraria from Bayer, Biogen, Merck, Mylan, Novartis, Roche, Sanofi-Genzyme and Teva.
Monica Margonireports grants and personal fees from Sanofi Genzyme, Merck Serono, Novartis and Almirall. She was awarded a MAGNIMS-ECTRIMS fellowship in 2020.
Paolo Gallo has been a consultant and member of Advisory Board for Biogen Italy, Sanofi-Genzyme, Merck-Serono, Almirall, Roche, Bristol-Meyer-Squibb and Novartis-Farma; has received funding for travel and speaker honoraria from Merck-Serono, Biogen Italy, Sanof-Genzyme, Novartis-Pharma, Roche, Almirall; has received research support from Biogen Italy, Merk-Serono, Sanofi Genzyme, Roche, Novartis.
Graziella Callari reports no disclosures.
Luigi Grimaldi has received travel funding and/or speaker honoraria from Biogen, Merck Serono, Novartis, Sanofi, and Teva and research support from Biogen and Merck Serono.
Giacomo Lus received personal compensation for speaking or consultancy from Biogen, Teva, Genzyme, Merck, Novartis, Almirall, Merz, and Ipsen.
Massimiliano Calabrese received speaker honoraria and consulting fees from Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, and Roche and received research support from the Progressive MS Alliance and the Italian Minister of Health.
Marta Simone reports no disclosures.
Marfia Girolama Alessandra is an Advisory Board member of Biogen Idec,Genzyme, Merck-Serono, Novartis, Teva and received honoraria forspeaking or consultation fees from Almirall, Bayer Schering, BiogenIdec, Merck Serono, Novartis, Sanofi-Genzyme, Roche, Mylan, Teva. Sheis the principal investigator in clinical trials for Biogen Idec,Merck Serono, Novartis, Roche, Sanofi-Genzyme, Teva.
Sarah Rasia reports no disclosures
Daniela Cargnelutti reports no disclosures.
Giancarlo Comi reports personal fees from Novartis, Teva Pharmaceutical Industries Ltd, Teva Italia Srl, Sanofi Genzyme, Genzyme Corporation, Genzyme Europe, Merck KGgA, Merck Serono SpA, Celgene Group, Biogen Idec, Biogen Italia Srl, F. Hoffman-La Roche, Roche SpA, Almirall SpA, Forward Pharma, Medday, Excemed.
Mauro Zaffaroni has received honoraria for consulting or lecturing and travel grants from: Alexion, Almirall, Biogen, Celgene, Janssen-Cilag, Merck, Novartis, Sanofi-Genzyme.
Collaborators
Maria Trojano has served on scientific Advisory boards for Biogen, Novartis, Roche, Merck , BMS and Genzyme; has received speaker honoraria from Biogen, Roche, Sanofi, Merck, Genzyme and Novartis; and has received research grants for her Institution from Biogen, Merck, Novartis and Roche.
Diego Centonze is an advisory board member for Almirall, Bayer, Biogen, GW Pharmaceuticals, Merck, Novartis, Roche, Sanofi, and Teva, and received honoraria for speaking or consultation fees from Almirall, Bayer, Biogen, GW Pharmaceuticals, Novartis, Roche, Sanofi, and Teva. He is also the principal investigator in clinical trials for Bayer, Biogen, Merck, Mitsubishi, Novartis, Roche, Sanofi, and Teva. His preclinical and clinical research was supported by grants from Bayer, Biogen, Celgene (BMS), Merck, Novartis, Roche, Sanofi, and Teva.
Ruggero Capra reports lecture fees and/or travel grants from Novartis, Biogen, Roche, Celgene and Merck.
Marco Capobianco received personal fees for advisory board and/or sponsored meeting from Biogen, Merck, Roche, Novartis, Celgene-Bristol, Janssen, Beck Dickinson, Alexion. He also received research grant from FISM, Roche, Novartis.
Alice Laroni received grants or contracts from Italian Ministry of University, Ministry of Health; received consulting fees from Merck, Biogen, Roche, Novartis, Bristol-Myers Squibb Pharma EEIG; received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Mercks, Biogen, Roche, Novartis, Bristol-Myers Squibb Pharma EEIG.
Antonio Uccelli received grants (to his Institution) from FISM, Biogen, Roche, Alexion, Merck Serono; participated on a Data Safety Monitoring Board or Advisory Board (to his Institution) for BD, Biogen, Iqvia, Sanofi, Roche, Alexion, Bristol Myers Squibb.
Antonio Gallo received personal compensation for speaking and consultancy from Biogen, Bristol Myers Squibb, Merck-Serono, Mylan, Novartis, Roche, Sanofi-Genzyme, and Teva.
Francesco Patti received personal compensation for serving on advisory board or speaking ad ECM events by Alexion, Almirall, Bayer, Biogen, Bristol Meyers&Squibb, Janssen, Merck, Novartis, Roche, Sanofi. He also received research grants by Almirall, Biogen, Merck, and Roche, FISM, MIUR and Reload Onlus Association.
Maura Chiara Danni reports no disclosures.
Claudio Gasperini has received compensation for consulting from Bayer HealthCare and Biogen and as a speaker for lectures from Biogen, Bayer HealthCare, Genzyme, Merck Serono, Novartis and Teva.
Gabriella Coniglio reports no disclosures.
Ethical approval
This study received approval from the ethical committee of the coordinating center (Multiple Sclerosis Center, Gallarate Hospital, ASST Valle Olona).
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baroncini, D., Ghezzi, A., Guaschino, C. et al. Long-term follow-up (up to 11 years) of an Italian pediatric MS cohort treated with Natalizumab: a multicenter, observational study. Neurol Sci 43, 6415–6423 (2022). https://doi.org/10.1007/s10072-022-06211-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06211-8