Abstract
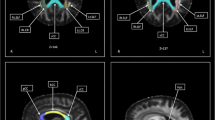
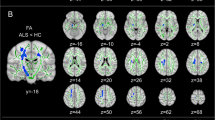
In this study, we used an automated segmentation of regions of interest and co-registration to diffusion tensor imaging (DTI) images to investigate whether microstructural abnormalities occur in gray structures of the frontal-subcortical circuits in patients with amyotrophic lateral sclerosis (ALS). Twenty-four patients with probable or definite sporadic ALS and 22 healthy controls were enrolled in the study. Thirteen out of 24 ALS patients and all of the control subjects underwent a detailed neuropsychological evaluation. DTI was performed to measure mean diffusivity (MD) and fractional anisotropy in the frontal cortex, caudate, putamen, globus pallidus, thalamus, amygdala and hippocampus. MD values of ALS patients were significantly higher in the frontal cortex (P = 0.023), caudate (P = 0.01), thalamus (P = 0.019), amygdala (P = 0.012) and hippocampus (P = 0.002) compared to controls. MD of these structures significantly correlated to a variable degree with neurological disability and neuropsychological dysfunctions. The increased MD values in several cortical and subcortical gray structures and their correlations with neuropsychological variables substantiate a multisystemic degeneration in ALS and suggest that dysfunctions of frontal–subcortical circuits could play a pivotal role in frontal impairment and behavioral symptoms in ALS patients.

Similar content being viewed by others
References
Rowland LP (1998) Diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 160(Suppl 1):6–24
Smith MC (1960) Nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 23:269–282
Bigio EH, Lipton AM, White CL 3rd, Dickson DW, Hirano A (2003) Frontotemporal and motor neurone degeneration with neurofilament inclusion bodies: additional evidence for overlap between FTD and ALS. Neuropathol Appl Neurobiol 29:239–253
Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE (2005) Prevalence and pattern of cognitive impairment in sporadic amyotrophic lateral sclerosis. Neurology 65:586–590
Talbot PR, Goulding PJ, Lloyd JJ, Snowden JS, Neary D, Testa HJ (1995) Inter-relation between “classic” motor neuron disease and frontotemporal dementia: neuropsychological and single photon emission computed tomography study. J Neurol Neurosurg Psychiatry 58:541–547
Thivard L, Pradat PF, Lehéricy S, Lacomblez L, Dormont D, Chiras J, Benali H, Meininger V (2007) Diffusion tension imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry 78:889–892
Chenevert TL, Brunberg JA, Pipe JG (1990) Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology 177:401–405
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusiontensor MRI. J Magn Reson B 111:209–219
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SC, Leigh PN (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058
Graham JM, Papadakis N, Evans J, Widjaja E, Romanowski CA, Paley MN, Wallis LI, Wilkinson ID, Shaw PJ, Griffiths PD (2004) Diffusion tensor imaging for the assessment of upper motor neuron integrity in ALS. Neurology 63:2111–2119
Toosy AT, Werring DJ, Orrell RW, Howard RS, King MD, Barker GJ, Miller DH, Thompson AJ (2003) Diffusion tensor imaging detects corticospinal tract involvement at multiple levels in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 74:1250–1257
Ciccarelli O, Behrens TE, Johansen-Berg H, Talbot K, Orrell RW, Howard RS, Nunes RG, Miller DH, Matthews PM, Thompson AJ, Smith SM (2009) Investigation of white matter pathology in ALS and PLS using tract-based spatial statistics. Hum Brain Mapp 30:615–624
Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR (2010) Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75:1645–1652
Cirillo M, Esposito F, Tedeschi G, Caiazzo G, Sagnelli A, Piccirillo G, Conforti R, Tortora F, Monsurrò MR, Cirillo S, Trojsi F (2012) Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol 33:1102–1108
Canu E, Agosta F, Riva N, Sala S, Prelle A, Caputo D, Perini M, Comi G, Filippi M (2011) The topography of brain microstructural damage in amyotrophic lateral sclerosis assessed using diffusion tensor MR imaging. AJNR Am J Neuroradiol 32:1307–1314
Sharma KR, Sheriff S, Maudsley A, Govind V (2013) Diffusion tensor imaging of basal ganglia and thalamus in amyotrophic lateral sclerosis. J Neuroimaging 23:368–374
Kawashima T, Doh-ura K, Kikuchi H, Iwaki T (2001) Cognitive dysfunction in patients with amyotrophic lateral sclerosis is associated with spherical or crescent-shaped ubiquitinated intraneuronal inclusions in the parahippocampal gyrus and amygdala, but not in the neostriatum. Acta Neuropathol 102:467–472
Takeda T, Uchihara T, Arai N, Mizutani T, Iwata M (2009) Progression of hippocampal degeneration in amyotrophic lateral sclerosis with or without memory impairment: distinction from Alzheimer disease. Acta Neuropathol 117:35–44
Anderson VE, Cairns NJ, Leigh PN (1995) Involvement of the amygdala, dentate and hippocampus in motor neuron disease. J Neurol Sci 129(Suppl):75–78
Alexander GE, DeLong MR, Strick PL (1986) Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 9:357–381
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Beck AT, Steer RA, Brown GK (1996) Manual for beck depression inventory-II. Psychological Corporation, San Antonio
Rey A (1958) Memorisation d’une serie de 15 mots en 5 repetitions. Presses Universitaries des France, Paris
Benton A, Hamsher KD (1989) Multilingual aphasia examination. AJA Associates, Iowa City
Nelson HE (1976) A modified card sorting test sensitive to frontal lobe defects. Cortex 12:313–324
Dubois B, Slachevsky A, Litvan I, Pillon B (2000) The FAB: a frontal assessment battery at bedside. Neurology 55:1621–1626
Cherubini A, Péran P, Caltagirone C, Sabatini U, Spalletta G (2009) Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage 48:29–36
Nicoletti G, Lodi R, Condino F, Tonon C, Fera F, Malucelli E, Manners D, Zappia M, Morgante L, Barone P, Barbiroli B, Quattrone A (2006) Apparent diffusion coefficient measurements of the middle cerebellar peduncle differentiate the Parkinson variant of MSA from Parkinson’s disease and progressive supranuclear palsy. Brain 129:2679–2687
Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G (1996) Diffusion tensor MR imaging of the human brain. Radiology 201:637–648
Senda J, Kato S, Kaga T, Ito M, Atsuta N, Nakamura T, Watanabe H, Tanaka F, Naganawa S, Sobue G (2011) Progressive and widespread brain damage in ALS: MRI voxel-based morphometry and diffusion tensor imaging study. Amyotroph Lateral Scler 12:59–69
Lillo P, Mioshi E, Burrell JR, Kiernan MC, Hodges JR, Hornberger M (2012) Grey and white matter changes across the amyotrophic lateral sclerosis-frontotemporal dementia continuum. PLoS ONE 7:e43993
Sharma KR, Saigal G, Maudsley AA, Govind V (2011) 1H MRS of basal ganglia and thalamus in amyotrophic lateral sclerosis. NMR Biomed 24:1270–1276
Al-Sarraj S, Maekawa S, Kibble M, Everall I, Leigh N (2002) Ubiquitin-only intraneuronal inclusion in the substantia nigra is a characteristic feature of motor neurone disease with dementia. Neuropathol Appl Neurobiol 28:120–128
Geser F, Martinez-Lage M, Robinson J, Uryu K, Neumann M, Brandmeir NJ, Xie SX, Kwong LK, Elman L, McCluskey L, Clark CM, Malunda J, Miller BL, Zimmerman EA, Qian J, Van Deerlin V, Grossman M, Lee VM, Trojanowski JQ (2009) Clinical and pathological continuum of multisystem TDP-43 proteinopathies. Arch Neurol 66:180–189
Geser F, Brandmeir NJ, Kwong LK, Martinez-Lage M, Elman L, McCluskey L, Xie SX, Lee VM, Trojanowski JQ (2008) Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol 65:636–641
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, van den Heuvel MP, van den Berg LH (2012) Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 83:383–388
D’Ambrosio A, Gallo A, Trojsi F, Corbo D, Esposito F, Cirillo M, Monsurró MR, Tedeschi G (2013) Frontotemporal cortical thinning in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. doi:10.3174/ajnr.A3753
DeLong MR, Wichmann T (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64:20–24
Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, Murphy J, Shoesmith C, Rosenfeld J, Leigh PN, Bruijn L, Ince P, Figlewicz D (2009) Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 10:131–146
Cummings JL (1995) Anatomic and behavioral aspects of frontal-subcortical circuits. Ann N Y Acad Sci 15(769):1–13
Bonelli RM, Cummings JL (2007) Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 9:141–151
Tekin S, Cummings JL (2002) Frontal-subcortical neuronal circuits and clinical neuropsychiatry: an update. J Psychosom Res 53:647–654
Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, Salvi F, Filippi M (2010) Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 31:1457–1461
Sato K, Aoki S, Iwata NK, Masutani Y, Watadani T, Nakata Y, Yoshida M, Terao Y, Abe O, Ohtomo K, Tsuji S (2010) Diffusion tensor tract-specific analysis of the uncinate fasciculus in patients with amyotrophic lateral sclerosis. Neuroradiology 52:729–733
Sarro L, Agosta F, Canu E, Riva N, Prelle A, Copetti M, Riccitelli G, Comi G, Filippi M (2011) Cognitive functions and white matter tract damage in amyotrophic lateral sclerosis: a diffusion tensor tractography study. AJNR Am J Neuroradiol 32:1866–1872
Sage CA, Peeters RR, Görner A, Robberecht W, Sunaert S (2007) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. Neuroimage 34:486–499
Tsujimoto M, Senda J, Ishihara T, Niimi Y, Kawai Y, Atsuta N, Watanabe H, Tanaka F, Naganawa S, Sobue G (2011) Behavioral changes in early ALS correlate with voxel-based morphometry and diffusion tensor imaging. J Neurol Sci 307:34–40
Li J, Pan P, Song W, Huang R, Chen K, Shang H (2012) A meta-analysis of diffusion tensor imaging studies in amyotrophic lateral sclerosis. Neurobiol Aging 33:1833–1838
Phukan J, Pender NP, Hardiman O (2007) Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol 6:994–1003
Christidi F, Zalonis I, Smyrnis N, Evdokimidis I (2012) Selective attention and the three-process memory model for the interpretation of verbal free recall in amyotrophic lateral sclerosis. J Int Neuropsychol Soc 18:809–818
Ludolph AC, Langen KJ, Regard M, Herzog H, Kempo B, Kurwert T (1992) Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand 85:81–89
Gallassi R, Montagna P, Ciardulli C, Lorusso S, Mussuto V, Stracciari A (1985) Cognitive impairment in motor neuron disease. Acta Neurol Scand 71:480–484
Moretti R, Torre P, Antonello RM, Carraro N, Cazzato G, Bava A (2002) Complex cognitive disruption in motor neuron disease. Dement Geriatr Cogn Disord 14:141–150
Ahn SW, Kim SH, Kim JE, Kim SM, Kim SH, Sung JJ, Lee KW, Hong YH (2011) Frontal assessment battery to evaluate frontal lobe dysfunction in ALS patients. Can J Neurol Sci 38:242–246
Stukovnik V, Zidar J, Podnar S, Repovs G (2010) Amyotrophic lateral sclerosis patients show executive impairments on standard neuropsychological measures and an ecologically valid motor-free test of executive functions. J Clin Exp Neuropsychol 32:1095–1109
Achi EY, Rudnicki SA (2012) ALS and frontotemporal dysfunction: a review. Neurol Res Int 2012:806306
Mioshi E, Lillo P, Yew B, Hsieh S, Savage S, Hodges JR, Kiernan MC, Hornberger M (2013) Cortical atrophy in ALS is critically associated with neuropsychiatric and cognitive changes. Neurology 80:1117–1123
Cerami C, Dodich A, Canessa N, Crespi C, Iannaccone S, Corbo M, Lunetta C, Consonni M, Scola E, Falini A, Cappa SF (2013) Emotional empathy in amyotrophic lateral sclerosis: a behavioural and voxel-based morphometry study. Amyotroph Lateral Scler Frontotemporal Degener. doi:10.3109/21678421.2013.785568
Passamonti L, Fera F, Tessitore A, Russo A, Cerasa A, Gioia CM, Monsurrò MR, Migliaccio R, Tedeschi G, Quattrone A (2013) Dysfunctions within limbic-motor networks in amyotrophic lateral sclerosis. Neurobiol Aging 34:2499–2509
Massman PJ, Sims J, Cooke N, Haverkamp LJ, Appel V, Appel SH (1996) Prevalence and correlates of neuropsychological deficits in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 61:450–455
Averill AJ, Kasarskis EJ, Segerstrom SC (2007) Psychological health in patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler 8:243–254
Atassi N, Cook A, Pineda CM, Yerramilli-Rao P, Pulley D, Cudkowicz M (2011) Depression in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 12:109–112
Jelsone-Swain L, Persad C, Votruba KL, Weisenbach SL, Johnson T, Gruis KL, Welsh RC (2012) The relationship between depressive symptoms, disease state, and cognition in amyotrophic lateral sclerosis. Front Psychol 3:542
Grossman AB, Woolley-Levine S, Bradley WG, Miller RG (2007) Detecting neurobehavioral changes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 8:56–61
Gibbons ZC, Richardson A, Neary D, Snowden JS (2008) Behaviour in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 9:67–74
Woolley SC, Zhang Y, Schuff N, Weiner MW, Katz JS (2011) Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph Lateral Scler 12:52–58
Eslinger PJ, Moore P, Antani S, Anderson C, Grossman M (2012) Apathy in frontotemporal dementia: behavioral and neuroimaging correlates. Behav Neurol 25:127–136
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Barbagallo, G., Nicoletti, G., Cherubini, A. et al. Diffusion tensor MRI changes in gray structures of the frontal-subcortical circuits in amyotrophic lateral sclerosis. Neurol Sci 35, 911–918 (2014). https://doi.org/10.1007/s10072-013-1626-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-013-1626-z