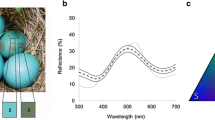
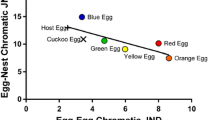
Abstract
At the core of recognition systems research are questions regarding how and when fitness-relevant decisions made. Studying egg-rejection behavior by hosts to reduce the costs of avian brood parasitism has become a productive model to assess cognitive algorithms underlying fitness-relevant decisions. Most of these studies focus on how cues and contexts affect hosts’ behavioral responses to foreign eggs; however, the timing of when the cues are perceived for egg-rejection decisions is less understood. Here, we focused the responses of American robins Turdus migratorius to model eggs painted with a thermochromic paint. This technique modified an egg’s color with predictably varying temperatures across incubation: at the onset of incubation, the thermochromic model egg was cold and perceptually similar to a static blue model egg (mimicking the robin’s own blue–green egg color), but by the end of an incubation bout, it was warm and similar to a static beige egg (mimicking the ground color of the egg of the robin’s brood parasite, the brown-headed cowbird Molothrus ater). Thermochromic eggs were rejected at statistically intermediate rates between those of the static blue (mostly accepted) and static beige (mostly rejected) model eggs. This implies that at the population level, egg-rejection relevant cues are not perceived solely when arriving to or solely when departing from the nest. We also found that robins rejected their own eggs more often when exposed to color-changing model eggs relative to static eggs, suggesting that recognizing variable foreign eggs entails costly rejection errors for this host species.



Similar content being viewed by others
References
Antonov A, Stokke BG, Moksnes A, Roskaft E (2008) Getting rid of the cuckoo Cuculus canorus egg: why do hosts delay rejection? Behav Ecol 19:100–107
Ban M, Moskat C, Barta Z, Hauber ME (2013) Simultaneous viewing of own and parasitic eggs is not required for foreign egg rejection by a cuckoo host. Behav Ecol 24:1014–1021
Batista G, Johnson JL, Dominguez E, Costa-Mattioli M, Pena JL (2016) Translational control of auditory imprinting and structural plasticity by eIF2α. eLife 5:e17197
Campbell DLM, Hauber ME (2010) Conspecific-only experience during development reduces the strength of heterospecific song discrimination in zebra finches (Taeniopygia guttata): a behavioural test of the optimal acceptance threshold hypothesis. J Ornithol 151:379–389
Caves EM, Green PA, Zipple MN, Peters S, Johnsen S, Nowicki S (2018) Categorical perception of colour signals in a songbird. Nature 560:365–367
Croston R, Hauber ME (2014) Spectral tuning and perceptual differences do not explain the rejection of brood parasitic eggs by American robins (Turdus migratorius). Behav Ecol Sociobiol 68:351–362
Croston R, Hauber ME (2015) Experimental shifts in intraclutch egg color variation do not affect egg rejection in a host of a non-egg-mimetic avian brood parasite. PLoS One 10:e0121213
Dainson M, Hauber ME, Lopez AV, Grim T, Hanley D (2017) Does contrast between eggshell ground and spot coloration affect egg rejection? Sci Nat 104:54
Davies NB, de Brooke ML (1989) An experimental study of co-evolution between the cuckoo, Cuculus canorus, and its host. I. Host egg discrimination. J Anim Ecol 58:207–224
Davies NB, de Brooke ML, Kacelnik A (1996) Recognition errors and probability of parasitism determine whether reed warblers should accept or reject mimetic cuckoo eggs. Proc R Soc Lond B 263:925–931
Grim T, Samas P, Moskat C, Kleven O, Honza M, Moksnes A, Roskaft E, Stokke BG (2011) Constraints on host choice: why do parasitic birds rarely exploit some common potential hosts? J Anim Ecol 80:508–518
Hanley D, Sulc M, Brennan P, Hauber ME, Grim T, Honza M (2016) Dynamic egg colour mimicry. Ecol Evol 6:4192–4202
Hanley D, Grim T, Igic B, Samas P, Lopez AV, Shawkey MD, Hauber ME (2017) Egg discrimination along a gradient of natural variation in eggshell coloration. Proc R Soc Lond B 284:20162592
Hanley D, Lopez AV, Fiorini VD, Reboreda JC, Grim T, Hauber ME (2019) Variation in multicomponent recognition cues alters egg rejection decisions: a test of the optimal acceptance threshold hypothesis. Philos Trans R Soc Lond B 374:20180195
Hauber ME (2003) Egg-capping is a cost paid by hosts of interspecific brood parasites. Auk 120:860–865
Hauber ME, Moskat C, Ban M (2006) Experimental shift in hosts’ acceptance threshold of inaccurate-mimic brood parasite eggs. Biol Lett 2:177–180
Hauber ME, Tong L, Ban M, Croston R, Grim T, Waterhouse GIN, Shawkey MD, Barron AB, Moskat C (2015) The value of artificial stimuli in behavioral research: making the case for egg rejection studies in avian brood parasitism. Ethology 121:521–528
Honza M, Prochazka P, Morongova K, Capek M, Jelinek V (2011) Do nest light conditions affect rejection of parasitic eggs? A test of the light environment hypothesis. Ethology 117:539–546
Igic B, Nunez V, Voss HU, Croston R, Aidala Z, Lopez AV, Van Tatenhove A, Holford ME, Shawkey MD, Hauber ME (2015) Using 3D printed eggs to examine the egg-rejection behaviour of wild birds. PeerJ 3:e965
Lahti DC (2015) The limits of artificial stimuli in behavioral research: the umwelt gamble. Ethology 121:529–537
Lang AK, Bollinger EK, Peer BD (2014) Effect of parasite-to-host egg ratio on egg rejection by a brown-headed cowbird host. Auk 131:694–701
Lorenzana JC, Sealy SG (2001) Fitness costs and benefits of cowbird egg ejection by gray catbirds. Behav Ecol 12:325–329
Luro AB, Hauber ME (2017) A test of the nest sanitation hypothesis for the evolution of foreign egg rejection in an avian brood parasite rejecter host species. Sci Nat 104:14
Luro A, Igic B, Croston R, Lopez AV, Shawkey MD, Hauber ME (2018) Which egg features predict egg rejection responses in American robins? Replicating Rothstein’s (1982) study. Ecol Evol 8:1673–1679
Lyon BE, Eadie JM (2017) Why do birds lay eggs in conspecifics’ nests? In: Soler M (ed) Avian brood parasitism. Springer Nature, Heidelberg, pp 105–123
Manna T, Moskat C, Hauber ME (2017) Ch. 24: Cognitive decision rules for egg rejection. In: Soler M (ed) Avian brood parasitism. Springer Nature, New York, pp 438–448
Mayani-Paras F, Kilner RM, Stoddard MC, Rodriguez C, Drummond H (2015) Behaviorally induced camouflage: a new mechanism of avian egg protection. Am Nat 186:E91–E97
Mendelson TC, Fitzpatrick CL, Hauber ME, Pence CH, Rodgriguez RL, Safran RJ, Stern CA, Stevens JR (2016) Cognitive phenotypes and the evolution of animal decisions. Trends Ecol Evol 11:850–859
Moreno J, Lobato E, Morales J (2011) Eggshell blue-green colouration fades immediately after oviposition: a cautionary note about measuring natural egg colours. Ornis Fenn 88:51–56
Pozgayova M, Prochazka P, Polacikova L, Honza M (2011) Closer clutch inspection—quicker egg ejection: timing of host responses toward parasitic eggs. Behav Ecol 22:46–51
Reeve HK (1989) The evolution of conspecific acceptance thresholds. Am Nat 133:407–435
Roncalli G, Soler M, Ruiz-Raya F, Serrano-Martin AJ, Ibanez-Alamo JD (2019) Predation risk affects egg-ejection but not recognition in blackbirds. Behav Ecol Sociobiol 73:56
Rothstein SI (1975a) An experimental and teleonomic investiga- tion of avian brood parasitism. Condor 77:250–271
Rothstein SI (1975b) Mechanisms of avian egg-recognition: do birds know their own eggs? Anim Behav 23:268–278
Rothstein SI (1982) Mechanisms of avian egg recognition: which egg parameters elicit responses by rejecter species? Behav Ecol Sociobiol 11:229–239
Scharf HM, Stenstrom K, Dainson M, Benson TJ, Fernandez-Juricic E, Hauber ME (2019) Mimicry-dependent lateralization in the visual inspection of foreign eggs by American robins. Biol Lett 15:20190351
Simons MJP, Verhulst S (2011) Zebra finch females prefer males with redder bills independent of song rate—a meta-analysis. Behav Ecol 22:755–762
Soler M, Ruiz-Raya F, Roncalli G, Ibanez-Alamo JD (2017) Relationships between egg-recognition and egg-ejection in a grasp-ejector species. PLoS One 12:e0166283
Stevens M, Troscianko J, Spottiswoode CN (2013) Repeated targeting of the same hosts by a brood parasite compromises host egg rejection. Nat Commun 4:2475
Stokke B, Honza M, Moksnes A, Roskaft E, Rudolfsen G (2002) Costs associated with recognition and rejection of parasitic eggs in two European passerines. Behaviour 139:629–644
Vanderhoff N, Pyle P, Patten MA, Sallabanks R, James FC (2016) America Robin. In: Rodewald PG (ed) The birds of North America. Cornell Lab of Ornithology, Ithaca
Vorobyev M, Osorio D (1998) Receptor noise as a determinant of colour thresholds. Proc R Soc Lond B 265:351–358
Acknowledgements
MEH, DH, and MD designed the study, DH and MD prepared the materials, MD and MEH collected field data, MD and AAL transcribed video observations, AL performed reflectance measurements and visual modelling, MEH conducted the statistical analyses, MEH wrote the first draft, and all authors contributed to the submitted versions of the manuscript. We thank the many landowners in Urbana who allowed us to work on their properties. The editor and the referees of the journal greatly improved on earlier versions of the manuscript.
Funding
Support for this project was provided by the Harley Jones Van Cleave Professorship at the University of Illinois (to MEH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
All authors declare no conflicts of interests.
Ethical approval
We followed the guidelines of the Animal Behavior Society for ethical treatment of research animals. The research was approved by the University of Illinois (IACUC #17049), the USA Department of the Interior (#23681), and the Illinois State Department of Natural Resources (#NH17.6099) issued to MEH.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hauber, M.E., Dainson, M., Luro, A. et al. When are egg-rejection cues perceived? A test using thermochromic eggs in an avian brood parasite host. Anim Cogn 22, 1141–1148 (2019). https://doi.org/10.1007/s10071-019-01306-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-019-01306-w