Abstract
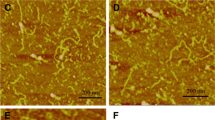
The effects of heat-induced rice bran protein (RBP) fibrils on structure and properties of solutions and gels in a complex system were investigated using transmission electron microscopy (TEM), atomic force microscopy (AFM), Congo red spectral analysis, and circular dichroism (CD). Planar and 3-dimensional images of RBP fibrils all revealed structural details. A Congo red spectral shift indicated fibril formation. Fibril secondary structural components exhibited differences at pH 2.0 and pH 7.0. The β-type was decreased with an increased pH. Rheological results exhibited shear thinning behavior for all solutions. Addition of fibrils to RBP solutions, which made the system complex, resulted in an order of magnitude increase in viscosity and shear stress. Adding fibrils to RBP solutions accelerated the kinetics of gel formation, resulting in an increase in gel strength. Scanning electron microscopy (SEM) images showed gel network structural differences with and without fibrils at different pH values.
Similar content being viewed by others
References
Akkermans C, Goot A.J, Venem P, Linden E, Boom RM. Cynthia A, Atze JG, Paul V, Erik L, Remko MB. Formation of fibrillar whey protein aggregates: Influence of heat and shear treatment, and resulting rheology. Food Hydrocolloid. 22: 1315–1325 (2008)
Akkermans C, Goot AJ, Venem P, Linden E, Remko MB. Properties of protein fibrils in whey protein isolate solutions: Microstructure, flow behaviour and gelation. Int. Dent. J. 18: 1034–1042 (2008)
Stephen RE, Saif UR, Geoffrey C. Denaturation and aggregation of β-lactoglobulin-a preliminary molecular dynamics study. Food Hydrocolloid. 21: 1081–1091 (2007)
Liu J, Tang CH. Heat-induced fibril assembly of vicilin at pH 2.0: Reaction kinetics, influence of ionic strength and protein concentration, and molecular mechanism. Food Res. Int. 51: 621–632 (2013)
Gao YZ, Xu HH, Ju TT, Zhao XH. The effect of limited proteolysis by different proteases on the formation of whey protein fibrils. J. Dairy. Sci. 96: 7383–7392 (2013)
Hamada D, Dobson CM. A kinetic study of beta-lactoglobulin amyloid fibril formation promoted by urea. Protein Sci. 11: 2417–2426 (2002)
Hill SE, Krebs B, Goodall DG, Howlett GJ, Dunstan DE. Shear flow induces amyloid fibril formation. Biomacromolecules 7: 10–13 (2006)
Keum EH, Lee SI, Oh SS. Effect of enzymatic hydrolysis of 7S globulin, a soybean protein, on its allergenicity and identification of its allergenic hydrolyzed fragments using SDS-PAGE. Food Sci. Biotechnol. 15: 128–132 (2006)
Nakano M, Shen JR, Kamino K. Self-assembling peptide inspired by a barnacle underwater adhesive protein. Biomacromolecules 8: 1830–1835 (2007)
Farahnaky A, Askari H, Majzoobi M, Mesbahi G. The impact of concentration, temperature and pH on dynamic rheology of psyllium gels. J. Food Eng. 100: 294–301 (2010)
Donato L, Garnier C, Novales B, Doublier JL. Gelation of globular protein in presence of low methoxyl pectin: effect of Na+ and/Ca2+ ions on rheology and microstructure of the systems. Food Hydrocolloid. 19: 549–556 (2005)
Zhang YH, Tang CH, Wen QB, Yang XQ, Li L, Deng WL. Thermal aggregation and gelation of kidney bean (Phaseolus vulgaris L.) protein isolate at pH 2.0: Influence of ionic strength. Food Hydrocolloid. 24: 266–274 (2010)
Karlsson AO, Ipsen R, Ardo Y. Influence of pH and NaCl on rheological properties of rennet-induced casein gels made from UF concentrated skim milk. Int. Dairy J. 17: 1053–1062 (2007)
Chanput W, Theerakulkait C, Nakai S. Antioxidative properties of partially purified barley hordein, rice bran protein fractions and their hydrolysates. J. Cereal Sci. 49: 422–428 (2009)
Davidson J, Mathieson J, Boyne AW. The use of automation in determining nitrogen by the Kjeldahl method, with final calculations by computer. Analyst 95: 181–193 (1970)
Tang CH, Zhang YH, Wen QB, Huang QR. Formation of amyloid fibrils from kidney bean 7S globulin (Phaseolin) at pH 2.0. J. Agr. Food Chem. 58: 8061–8068 (2010)
Weijers M, Sagis LMC, Veerman C, Sperber B, van der Linden E. Rheology and structure of ovalbumin gels at low pH and low ionic strength. Food Hydrocolloid. 16: 269–276 (2002)
Loveday SM, Wang XL, Rao MA, Anema SG, Singh H. β-Lactoglobulin nanofibrils: Effect of temperature on fibril formation kinetics, fibril morphology and the rheological properties of fibril dispersions. Food Hydrocolloid. 27: 242–249 (2012)
Tang CH, Wang SS, Huang QR. Improvement of heat-induced fibril assembly of soy β-conglycinin (7S Globulins) at pH 2.0 through electrostatic screening. Food Res. Int. 46: 229–236 (2012)
Sudeshna G, Nitin KP, Sambuddha S, Debi RT, Swagata D. Binding of hen egg white lysozyme fibrils with nucleic acids. J. Photoch. Photobio. B 127: 52–60 (2013)
Rana A, Gupta TP, Bansal S, Kundu B. Formation of amyloid fibrils by bovine carbonic anhydrase. Biochim. Biophys. Acta 1784: 930–35 (2008)
Melanie R. Nilsson. Techniques to study amyloid fibril formation in vitro. Methods 34: 151–160 (2004)
Martino C, Fabrizio C, Christopher MD. Amyloid fibril formation can proceed from different conformations of a partially unfolded protein. Biophys. J. 89: 4201–4210 (2005)
Khodarahmi R, Beyrami M, Soori H. Appraisal of casein’s inhibitory effects on aggregation accompanying carbonic anhydrase refolding and heat-induced ovalbumin fibrillogenesis. Arch. Biochem. Biophys. 477: 67–76 (2008)
Kelly SM, Price NC. The application of circular dichroism to studies of protein folding and unfolding. Biochim. Biophys. Acta 1338: 161–185 (1997)
Pearce FG, Mackintosh SH, Gerrard JA. Formation of amyloid-like fibrils by ovalbumin and related proteins under conditions relevant to food processing. J. Agr. Food Chem. 55: 318–322 (2007)
Narasimha S, Robert W. Estimation of protein secondary structure from circular dichroism spectra: comparison of CONTIN, SELCON, and CDSSTR methods with an expanded reference set. Anal. Biochem. 287: 252–260 (2000)
Akkermans C, Goot AJ, Venem P, Gruppen H, Vereijken JM, Linden E, Boom RM. Micrometer-sized fibrillar protein aggregates from soy glycinin and soy protein isolate. J. Agr. Food Chem. 55: 9877–9882 (2007)
Kastner H, Einhorn SU, Senge B. New parameters for the examination of the pectin gelation process. Roy. Soc. Ch. 16: 191–197 (2012)
Choi YS, Choi JH, Han DJ, Kim HY, Lee MA, Kim HW, Jeong JY, Kim CJ. Effects of rice bran fiber on heat-induced gel prepared with pork salt-soluble meat proteins in model system. Meat Sci. 88: 59–66 (2011)
Matzinos P, Álvarez R. Effect of ionic strength on rinsing and alkaline cleaning of ultrafiltration inorganic membranes fouled with whey proteins. J. Membrane Sci. 208: 23–30 (2002)
He JS, Azuma N, Yang HW. Effects of pH and ionic strength on the rheology and microstructure of a pressure-induced whey protein gel. Int. Dairy J. 20: 89–95 (2010)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, YH., Huang, LH. Effect of heat-induced formation of rice bran protein fibrils on morphological structure and physicochemical properties in solutions and gels. Food Sci Biotechnol 23, 1417–1423 (2014). https://doi.org/10.1007/s10068-014-0194-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-014-0194-1