Abstract
Introduction
Celastrol is a promising therapeutic agent for the treatment of osteoarthritis (OA). However, the mechanism of action of celastrol is unclear. This study was aiming to identify the potential function of celastrol on OA and determine its underlying mechanism.
Method
Celastrol targets were collected from web database searches and literature review, while pathogenic OA targets were obtained from Online Mendelian Inheritance in Man (OMIM) and GeneCards databases. Transcriptomics data was sequenced using an Illumina HiSeq 4000 platform. Celastrol-OA overlapping genes were then identified followed by prediction of the potential function and signaling pathways associated with celastrol using gene ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. A celastrol-target network was constructed to identify the candidate core targets of celastrol. The predictions were then validated by performing molecular docking and molecular dynamics simulation studies.
Results
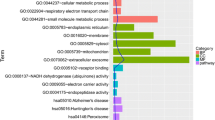
In total, 96 genes were identified as the putative celastrol targets for treatment of OA. These genes were possibly involved in cell phenotype changes including response to lipopolysaccharide and oxidative stress as well as in cell apoptosis and aging. The genes also induced the mTOR pathway and AGE-RAGE signaling pathway at the intracellular level. Additionally, results indicated that 13 core targets including mTOR, TP53, MMP9, EGFR, CCND1, MAPK1, STAT3, VEGFA, CASP3, TNF, MYC, ESR1, and PTEN were likely direct targets of celastrol in OA. Finally, mTOR was determined as the most likely therapeutic target of celastrol in OA.
Conclusion
This study provides a basic understanding and novel insight into the potential mechanism of celastrol against OA.
Key Points • Our study provides a strong indication that further study of celastrol therapy in OA is required. • mTOR is the most likely therapeutic target of celastrol in OA. |





Similar content being viewed by others
Data availability
The associated raw sequencing data in this study has been deposited in the Sequence Read Archive (SRA) under accession ID PRJNA602231. All other data generated or analyzed during this study are included within the article and its supplementary files.
Abbreviations
- BP:
-
Biological process
- CC:
-
Cellular component
- DMOADs:
-
Disease-modifying osteoarthritis drugs
- GO:
-
Gene ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- MD:
-
Molecular dynamics
- MF:
-
Molecular function
- OMIM:
-
Online Mendelian Inheritance in Man
- OA:
-
Osteoarthritis
- PPI:
-
Protein-protein interaction
- PDB:
-
Protein Data Bank
- RMSD:
-
Root-mean-square deviation
- SRA:
-
Sequence Read Archive
- SEArch:
-
Similarity ensemble approach
- TCMSP:
-
Traditional Chinese medicine systems pharmacology
References
Oo WM, Yu SP, Daniel MS, Hunter DJ (2018) Disease-modifying drugs in osteoarthritis: current understanding and future therapeutics. Expert Opin Emerg Drugs 23(4):331–347. https://doi.org/10.1080/14728214.2018.1547706
Karsdal MA, Michaelis M, Ladel C, Siebuhr AS, Bihlet AR, Andersen JR, Guehring H, Christiansen C, Bay-Jensen AC, Kraus VB (2016) Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: lessons learned from failures and opportunities for the future. Osteoarthr Cartil 24(12):2013–2021. https://doi.org/10.1016/j.joca.2016.07.017
Venkatesha SH, Dudics S, Astry B, Moudgil KD (2016) Control of autoimmune inflammation by celastrol, a natural triterpenoid. Pathog Dis 74(6):ftw059. https://doi.org/10.1093/femspd/ftw059
Li J, Hao J (2019) Treatment of neurodegenerative diseases with bioactive components of Tripterygium wilfordii. Am J Chin Med 47(4):769–785. https://doi.org/10.1142/s0192415x1950040x
Liu J, Lee J, Salazar Hernandez MA, Mazitschek R, Ozcan U (2015) Treatment of obesity with celastrol. Cell 161(5):999–1011. https://doi.org/10.1016/j.cell.2015.05.011
Wang W, Ha C, Lin T, Wang D, Wang Y, Gong M (2018) Celastrol attenuates pain and cartilage damage via SDF-1/CXCR4 signalling pathway in osteoarthritis rats. J Pharm Pharmacol 70(1):81–88. https://doi.org/10.1111/jphp.12835
Ding QH, Cheng Y, Chen WP, Zhong HM, Wang XH (2013) Celastrol, an inhibitor of heat shock protein 90beta potently suppresses the expression of matrix metalloproteinases, inducible nitric oxide synthase and cyclooxygenase-2 in primary human osteoarthritic chondrocytes. Eur J Pharmacol 708(1-3):1–7. https://doi.org/10.1016/j.ejphar.2013.01.057
Ruiz-Romero C, Rego-Perez I, Blanco FJ (2018) What did we learn from ‘omics’ studies in osteoarthritis. Curr Opin Rheumatol 30(1):114–120. https://doi.org/10.1097/bor.0000000000000460
Hopkins AL (2008) Network pharmacology: the next paradigm in drug discovery. Nat Chem Biol 4(11):682–690. https://doi.org/10.1038/nchembio.118
Berger SI, Iyengar R (2009) Network analyses in systems pharmacology. Bioinformatics 25(19):2466–2472. https://doi.org/10.1093/bioinformatics/btp465
Wang Y, Xiao J, Suzek TO, Zhang J, Wang J, Bryant SH (2009) PubChem: a public information system for analyzing bioactivities of small molecules. Nucleic Acids Res 37(Web Server issue):W623–W633. https://doi.org/10.1093/nar/gkp456
Gfeller D, Michielin O, Zoete V (2013) Shaping the interaction landscape of bioactive molecules. Bioinformatics 29(23):3073–3079. https://doi.org/10.1093/bioinformatics/btt540
Keiser MJ, Roth BL, Armbruster BN, Ernsberger P, Irwin JJ, Shoichet BK (2007) Relating protein pharmacology by ligand chemistry. Nat Biotechnol 25(2):197–206. https://doi.org/10.1038/nbt1284
Ru J, Li P, Wang J, Zhou W, Li B, Huang C, Li P, Guo Z, Tao W, Yang Y, Xu X, Li Y, Wang Y, Yang L (2014) TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. J Cheminform 6:13. https://doi.org/10.1186/1758-2946-6-13
Cascao R, Fonseca JE, Moita LF (2017) Celastrol: a spectrum of treatment opportunities in chronic diseases. Front Med (Lausanne) 4:69. https://doi.org/10.3389/fmed.2017.00069
Chen SR, Dai Y, Zhao J, Lin L, Wang Y, Wang Y (2018) A mechanistic overview of triptolide and celastrol, natural products from Tripterygium wilfordii Hook F. Front Pharmacol 9:104. https://doi.org/10.3389/fphar.2018.00104
Hu M, Luo Q, Alitongbieke G, Chong S, Xu C, Xie L, Chen X, Zhang D, Zhou Y, Wang Z (2017) Celastrol-induced Nur77 interaction with TRAF2 alleviates inflammation by promoting mitochondrial ubiquitination and autophagy. Mol Cell 66(1):141–153.e146. https://doi.org/10.1016/j.molcel.2017.03.008
The UniProt Consortium (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47(D1):D506–d515. https://doi.org/10.1093/nar/gky1049
Zhang J, Li C, Zheng Y, Lin Z, Zhang Y, Zhang Z (2017) Inhibition of angiogenesis by arsenic trioxide via TSP-1-TGF-β1-CTGF-VEGF functional module in rheumatoid arthritis. Oncotarget 8(43):73529–73546. https://doi.org/10.18632/oncotarget.19867
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D (2016) The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics 54:1.30.31–31.30.33. https://doi.org/10.1002/cpbi.5
Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A (2015) OMIM.org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res 43(Database issue):D789–D798. https://doi.org/10.1093/nar/gku1205
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV (2019) STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 47(D1):D607–d613. https://doi.org/10.1093/nar/gky1131
Nacher JC, Schwartz JM (2008) A global view of drug-therapy interactions. BMC Pharmacol 8:5. https://doi.org/10.1186/1471-2210-8-5
Kitchen DB, Decornez H, Furr JR, Bajorath J (2004) Docking and scoring in virtual screening for drug discovery: methods and applications. Nat Rev Drug Discov 3(11):935–949. https://doi.org/10.1038/nrd1549
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The Protein Data Bank. Nucleic Acids Res 28(1):235–242. https://doi.org/10.1093/nar/28.1.235
Liu DD, Zhang BL, Yang JB, Zhou K (2020) Celastrol ameliorates endoplasmic stress-mediated apoptosis of osteoarthritis via regulating ATF-6/CHOP signalling pathway. J Pharm Pharmacol 72(6):826–835. https://doi.org/10.1111/jphp.13250
Li X, Wei W, Zhao Z, Lv S (2019) Tripterine up-regulates miR-223 to alleviate lipopolysaccharide-induced damage in murine chondrogenic ATDC5 cells. Int J Immunopathol Pharmacol 33:2058738418824521. https://doi.org/10.1177/2058738418824521
Du Z, Zhang W, Wang S, Zhang J, He J, Wang Y, Dong Y, Huo M (2019) Celastrol protects human retinal pigment epithelial cells against hydrogen peroxide mediated oxidative stress, autophagy, and apoptosis through sirtuin 3 signal pathway. J Cell Biochem 120(6):10413–10420. https://doi.org/10.1002/jcb.28326
Xu XJ, Zhao WB, Feng SB, Sun C, Chen Q, Ni B, Hu HY (2017) Celastrol alleviates angiotensin II-mediated vascular smooth muscle cell senescence via induction of autophagy. Mol Med Rep 16(5):7657–7664. https://doi.org/10.3892/mmr.2017.7533
Li L, Wang B, Li Y, Li L, Dai Y, Lv G, Wu P, Li P (2020) Celastrol regulates bone marrow mesenchymal stem cell fate and bone-fat balance in osteoporosis and skeletal aging by inducing PGC-1α signaling. Aging (Albany NY) 12(17):16887–16898. https://doi.org/10.18632/aging.103590
Sherwood J (2019) Osteoarthritis year in review 2018: biology. Osteoarthr Cartil 27(3):365–370. https://doi.org/10.1016/j.joca.2018.10.005
Zhang Y, Geng C, Liu X, Li M, Gao M, Liu X, Fang F, Chang Y (2017) Celastrol ameliorates liver metabolic damage caused by a high-fat diet through Sirt1. Mol Metab 6(1):138–147. https://doi.org/10.1016/j.molmet.2016.11.002
Allen SD, Liu YG, Kim T, Bobbala S, Yi S, Zhang X, Choi J, Scott EA (2019) Celastrol-loaded PEG-b-PPS nanocarriers as an anti-inflammatory treatment for atherosclerosis. Biomater Sci 7(2):657–668. https://doi.org/10.1039/c8bm01224e
Nie Y, Fu C, Zhang H, Zhang M, Xie H, Tong X, Li Y, Hou Z, Fan X, Yan M (2020) Celastrol slows the progression of early diabetic nephropathy in rats via the PI3K/AKT pathway. BMC Complement Med Ther 20(1):321. https://doi.org/10.1186/s12906-020-03050-y
Li X, Zhu G, Yao X, Wang N, Hu R, Kong Q, Zhou D, Long L, Cai J, Zhou W (2018) Celastrol induces ubiquitin-dependent degradation of mTOR in breast cancer cells. Onco Targets Ther 11:8977–8985. https://doi.org/10.2147/ott.S187315
Zhang Y, Vasheghani F, Li YH, Blati M, Simeone K, Fahmi H, Lussier B, Roughley P, Lagares D, Pelletier JP, Martel-Pelletier J, Kapoor M (2015) Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis 74(7):1432–1440. https://doi.org/10.1136/annrheumdis-2013-204599
Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F (2003) Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426(6967):620. https://doi.org/10.1038/426620a
Pal B, Endisha H, Zhang Y, Kapoor M (2015) mTOR: a potential therapeutic target in osteoarthritis? Drugs R D 15(1):27–36. https://doi.org/10.1007/s40268-015-0082-z
Lee JH, Choi KJ, Seo WD, Jang SY, Kim M, Lee BW, Kim JY, Kang S, Park KH, Lee YS, Bae S (2011) Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int J Mol Med 27(3):441–446. https://doi.org/10.3892/ijmm.2011.601
Zhang X, Yang J, Chen M, Li L, Huan F, Li A, Liu Y, Xia Y, Duan JA, Ma S (2016) Metabolomics profiles delineate uridine deficiency contributes to mitochondria-mediated apoptosis induced by celastrol in human acute promyelocytic leukemia cells. Oncotarget 7(29):46557–46572. https://doi.org/10.18632/oncotarget.10286
Ito K, Maruyama Z, Sakai A, Izumi S, Moriishi T, Yoshida CA, Miyazaki T, Komori H, Takada K, Kawaguchi H, Komori T (2014) Overexpression of Cdk6 and Ccnd1 in chondrocytes inhibited chondrocyte maturation and caused p53-dependent apoptosis without enhancing proliferation. Oncogene 33(14):1862–1871. https://doi.org/10.1038/onc.2013.130
Jeon OH, Kim C, Laberge RM, Demaria M, Rathod S, Vasserot AP, Chung JW, Kim DH, Poon Y, David N, Baker DJ, van Deursen JM, Campisi J, Elisseeff JH (2017) Local clearance of senescent cells attenuates the development of post-traumatic osteoarthritis and creates a pro-regenerative environment. Nat Med 23(6):775–781. https://doi.org/10.1038/nm.4324
Jimi E, Fei H, Nakatomi C (2019) NF-κB signaling regulates physiological and pathological chondrogenesis. Int J Mol Sci 20(24). https://doi.org/10.3390/ijms20246275
Code availability
Not applicable.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81771748) and Harbin Technology Bureau Research Fund (2017RAQXJ196).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics approval
Ethical approval was obtained from the First Affiliated Hospital of Harbin Medical University.
Consent to participate
Written informed was acquired from each participant before surgery.
Consent for publication
Informed consent for publication was obtained from each study participant and each patient.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dai, S., Wang, H., Wang, M. et al. Comparative transcriptomics and network pharmacology analysis to identify the potential mechanism of celastrol against osteoarthritis. Clin Rheumatol 40, 4259–4268 (2021). https://doi.org/10.1007/s10067-021-05726-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05726-3