Abstract
Introduction
We compared the performance of a fluorescence lateral flow assay (ichroma™ IGRA-TB) with the QuantiFERON-TB Gold PLUS (QFT-PLUS) for the diagnosis of latent tuberculosis infection (LTBI) in patients with immune-mediated inflammatory diseases (IMID) prior to receiving biologics therapy.
Method
The comparability of the ichroma™ IGRA-TB assay with the QFT-PLUS assay for the diagnosis of LTBI was determined in prospectively enrolled patients with IMID prior to receiving biologics between August 2018 and October 2019. To determine the best cut-off value of the ichroma™ IGRA-TB, an ROC curve analysis was performed.
Results
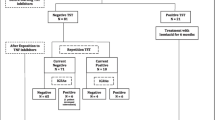
Patients with IMID (n = 145) had inflammatory bowel disease (n = 83; 57.2%), rheumatoid arthritis (n = 44; 30.3%), or spondyloarthropathy (n = 18; 12.4%). The median age was 40.5 (interquartile range: 27.0–56.0), 72 (49.7%) were men, and 140 (96.6%) received BCG vaccination. With the manufacturer-recommended cut-off values, 11 (7.6%) and 20 (13.8%) patients showed positive results with the ichroma™ IGRA-TB and QFT-PLUS tests, respectively. The overall agreement between the two tests was 91.0% with a Cohen’s kappa value of 0.535 (95% confidence interval: 0.317–0.754). ROC curve analysis of the QFT-PLUS results showed that a cut-off value of > 0.21 IU/mL would improve the performance of the ichroma™ IGRA-TB. Using the new cut-off value, the concordance rate was improved to 93.1% with a Cohen’s kappa value of 0.668 (95% confidence interval: 0.478–0.858).
Conclusions
The ichroma™ IGRA-TB could be used as a point-of-care test for LTBI screening in IMID patients before starting biologics, especially in resource-limited settings.
Key Points • The ichroma™ IGRA-TB is an automated fluorescence lateral flow assay–based IGRA. • The test has advantages like short turn-around time, low-cost, and ease of use. • The ichroma™ IGRA-TB showed high agreement with the QuantiFERON-TB Gold In-Tube in patients with chronic immune-mediated inflammatory diseases before starting biologics. |


Similar content being viewed by others
Data availability
Not applicable.
References
(2019) Global tuberculosis report 2019, Vol. 2020. Geneva: World Health Organization
Getahun H, Matteelli A, Chaisson RE, Raviglione M (2015) Latent Mycobacterium tuberculosis infection. N Engl J Med 372:2127–2135
Houben RM, Dodd PJ (2016) The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 13:e1002152
Comstock GW, Livesay VT, Woolpert SF (1974) The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 99:131–138
Vynnycky E, Fine PE (2000) Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol 152:247–263
Trauer JM, Moyo N, Tay EL, Dale K, Ragonnet R, McBryde ES et al (2016) Risk of active tuberculosis in the five years following infection . . . 15%? Chest 149:516–525
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM (2001) Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med 345:1098–1104
Gomez-Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD (2003) Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active-surveillance report. Arthritis Rheum 48:2122–2127
Seong SS, Choi CB, Woo JH, Bae KW, Joung CL, Uhm WS, Kim TH, Jun JB, Yoo DH, Lee JT, Bae SC (2007) Incidence of tuberculosis in Korean patients with rheumatoid arthritis (RA): effects of RA itself and of tumor necrosis factor blockers. J Rheumatol 34:706–711
Kim HW, Park JK, Yang JA, Yoon YI, Lee EY, Song YW, Kim HR, Lee EB (2014) Comparison of tuberculosis incidence in ankylosing spondylitis and rheumatoid arthritis during tumor necrosis factor inhibitor treatment in an intermediate burden area. Clin Rheumatol 33:1307–1312
(2020) WHO consolidated guideline on tuberculosis: tuberculosis preventive treatment, Vol. 2020. Geneva: World Health Organization. Licence: CC BY-NC-SA 3.0 IGO
Goletti D, Sanduzzi A, Delogu G (2014) Performance of the tuberculin skin test and interferon-gamma release assays: an update on the accuracy, cutoff stratification, and new potential immune-based approaches. J Rheumatol Suppl 91:24–31
Mazurek GH, LoBue PA, Daley CL, Bernardo J, Lardizabal AA, Bishai WR, Iademarco MF, Rothel JS (2001) Comparison of a whole-blood interferon gamma assay with tuberculin skin testing for detecting latent Mycobacterium tuberculosis infection. Jama 286:1740–1747
Wang L, Turner MO, Elwood RK, Schulzer M, FitzGerald JM (2002) A meta-analysis of the effect of Bacille Calmette Guerin vaccination on tuberculin skin test measurements. Thorax 57:804–809
Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sasaki Y, Mazurek GH, Tsuyuguchi I (2004) Specific detection of tuberculosis infection: an interferon-gamma-based assay using new antigens. Am J Respir Crit Care Med 170:59–64
Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, Yim JJ (2005) Discrepancy between the tuberculin skin test and the whole-blood interferon gamma assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. Jama 293:2756–2761
O’Garra A, Redford PS, McNab FW, Bloom CI, Wilkinson RJ, Berry MP (2013) The immune response in tuberculosis. Annu Rev Immunol 31:475–527
Redelman-Sidi G, Sepkowitz KA (2013) IFN-gamma release assays in the diagnosis of latent tuberculosis infection among immunocompromised adults. Am J Respir Crit Care Med 188:422–431
Go U, Park M, Kim U-N, Lee S, Han S, Lee J, Yang J, Kim J, Park S, Kim Y, Yoo H, Cha J, Park W, Kang H, Kim H, Park G, Kim M, Park O, Son H, Cho E, Na K, Kwon Y, Lee Y, Lee KH, Jeong E, Lee D, Yang BG, Jeon BY, Lee JK, Korea Tuberculosis Epidemic Investigation Service (2018) Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Mycobact Dis 11:28–36
Barcellini L, Borroni E, Brown J, Brunetti E, Campisi D, Castellotti PF, Codecasa LR, Cugnata F, di Serio C, Ferrarese M, Goletti D, Lipman M, Rancoita PMV, Russo G, Tadolini M, Vanino E, Cirillo DM (2016) First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur Respir J 48:1411–1419
Moon HW, Gaur RL, Tien SS, Spangler M, Pai M, Banaei N (2017) Evaluation of QuantiFERON-TB Gold-Plus in health care workers in a low-incidence setting. J Clin Microbiol 55:1650–1657
Pieterman ED, Liqui Lung FG, Verbon A, Bax HI, Ang CW, Berkhout J, Blaauw G, Brandenburg A, van Burgel ND, Claessen A, van Dijk K, Heron M, Hooghiemstra M, Leussenkamp-Hummelink R, van Lochem E, van Loo IHM, Mulder B, Ott A, Pontesilli O, Reuwer A, Rombouts P, Saegeman V, Scholing M, Vainio S, de Steenwinkel JEM (2018) A multicentre verification study of the QuantiFERON((R))-TB Gold Plus assay. Tuberculosis (Edinb) 108:136–142
Theel ES, Hilgart H, Breen-Lyles M, McCoy K, Flury R, Breeher LE et al (2018) Comparison of the QuantiFERON-TB Gold Plus and QuantiFERON-TB Gold In-Tube interferon gamma release assays in patients at risk for tuberculosis and in health care workers. J Clin Microbiol 56:e00614–e00618
Venkatappa TK, Punnoose R, Katz DJ, Higgins MP, Banaei N, Graviss EA et al (2019) Comparing QuantiFERON-TB Gold Plus with other tests to diagnose Mycobacterium tuberculosis infection. J Clin Microbiol 57:e00985–e00919
Kim SH, Jo KW, Shim TS (2020) QuantiFERON-TB Gold PLUS versus QuantiFERON- TB Gold In-Tube test for diagnosing tuberculosis infection. Korean J Intern Med 35:383–391
Won D, Park JY, Kim HS, Park Y (2020) Comparative Results of QuantiFERON-TB Gold In-Tube and QuantiFERON-TB Gold Plus assays for detection of tuberculosis infection in clinical samples. J Clin Microbiol 58:e01854–e01819
Hur YG, Hong JY, Choi DH, Kim A, Park SY, Kwon M, Kang K, Lee JM, Dockrell HM, Lee Y, Joo H, Cho SN (2019) A feasibility study for diagnosis of latent tuberculosis infection using an IGRA Point-of-Care Platform in South Korea. Yonsei Med J 60:375–380
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Metcalfe JZ, Cattamanchi A, McCulloch CE, Lew JD, Ha NP, Graviss EA (2013) Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am J Respir Crit Care Med 187:206–211
Jonsson J, Westman A, Bruchfeld J, Sturegård E, Gaines H, Schön T (2017) A borderline range for Quantiferon Gold In-Tube results. PLoS One 12:e0187313
(2020) Korean guidelines for tuberculosis, 4th ed, Vol. 2020. Cheongju: Korea Centers for Disease Control and Prevention
Code availability
Not applicable.
Funding
This work was supported in part by the Korea Health Technology R&D Project (HI17C1000), through the Korea Health Industry Development Institute, from the Ministry of Health & Welfare, South Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Institutional Review Board of Asan Medical Center (IRB No.: 2017-1303). All participants provided written informed consent.
Consent to participate
Obtained.
Consent to publication
Obtained.
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, H.H., Choi, D.H., Kim, JR. et al. Evaluation of a lateral flow assay–based IFN-γ release assay as a point-of-care test for the diagnosis of latent tuberculosis infection. Clin Rheumatol 40, 3773–3781 (2021). https://doi.org/10.1007/s10067-021-05663-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-021-05663-1