Abstract
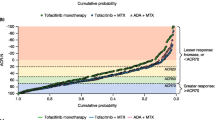
Approximately 30% of patients with rheumatoid arthritis receiving biological disease-modifying antirheumatic drugs (bDMARDs) take them as monotherapy. Although etanercept (ETN) monotherapy has been evaluated in clinical trials, data in the real-world setting are sparse. We compared the efficacy and safety of ETN, given alone or in combination with methotrexate (MTX), in routine clinical practice. This was a subanalysis of patients who received either ETN alone or ETN + MTX during a 52-week prospective, observational study conducted at 329 German centers. The primary endpoint was “Funktionsfragebogen Hannover” (Hannover Functional Ability Questionnaire [FFbH]; low FFbH = worse function) functional remission at week 26 and week 52. Secondary endpoints included the 28-joint count Disease Activity Score (DAS28), DAS28 remission (DAS28 < 2.6), and adverse events (AEs). Participating centers applied ETN monotherapy in 43.1% of patients and ETN + MTX in 56.9%. A smaller proportion of patients achieved FFbH functional remission with ETN vs ETN + MTX (31.9%, 95% confidence interval [CI] 29.1–34.9% vs 39.8%, 37.2–42.5%, respectively; p < 0.001) at 26 weeks and at 52 weeks (38.4%, 35.1–41.7% vs 44.3%, 41.5–47.2%, respectively; p = 0.007). After 52 weeks, the mean DAS28 (±SD) decreased from 5.5 ± 1.3 to 3.4 ± 1.4 (ETN) vs 5.3 ± 1.3 to 3.2 ± 1.3 (ETN + MTX) and DAS28 remission was achieved by 32.5% (95% CI 29.0–36.1%) of patients with ETN vs 38.8% (35.8–41.9%; p = 0.007) with ETN + MTX. Overall, 20.6 (ETN) and 19.7% (ETN + MTX) of patients reported treatment-related AEs. Patients received ETN monotherapy almost as often as ETN + MTX. ETN + MTX appeared marginally more effective than ETN monotherapy in some, but not all, outcomes measured.




Similar content being viewed by others
References
European Medicines Agency (2000) Enbrel summary of product characteristics http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000262/WC500027361.pdf. Accessed 3 March 2015
Smolen JS, Landewé R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Singh JA, Furst DE, Bharat A et al (2012) 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 64:625–639
Bathon JM, Martin RW, Fleischmann RM et al (2000) A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 343:1586–1593
Cannon GW, Wang BC, Park GS et al (2013) Remission in rheumatoid arthritis patients treated with etanercept monotherapy: clinical practice and clinical trial experience. Clin Exp Rheumatol 31:919–925
Genovese MC, Bathon JM, Fleischmann RM et al (2005) Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol 32:1232–1242
Genovese MC, Bathon JM, Martin RW et al (2002) Etanercept versus methotrexate in patients with early rheumatoid arthritis: two-year radiographic and clinical outcomes. Arthritis Rheum 46:1443–1450
Takeuchi T, Miyasaka N, Zang C et al (2013) A phase 3 randomized, double-blind, multicenter comparative study evaluating the effect of etanercept versus methotrexate on radiographic outcomes, disease activity, and safety in Japanese subjects with active rheumatoid arthritis. Mod Rheumatol 23:623–633
Emery P, Breedveld F, van der Heijde D et al (2010) Two-year clinical and radiographic results with combination etanercept-methotrexate therapy versus monotherapy in early rheumatoid arthritis: a two-year, double-blind, randomized study. Arthritis Rheum 62:674–682
Emery P, Breedveld FC, Hall S et al (2008) Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 372:375–382
Klareskog L, van der Heijde D, de Jager JP et al (2004) Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet 363:675–681
Machado DA, Guzman RM, Xavier RM et al (2014) Open-label observation of addition of etanercept versus a conventional disease-modifying antirheumatic drug in subjects with active rheumatoid arthritis despite methotrexate therapy in the Latin American region. J Clin Rheumatol 20:25–33
Pope JE, Haraoui B, Thorne JC et al (2014) The Canadian methotrexate and etanercept outcome study: a randomised trial of discontinuing versus continuing methotrexate after 6 months of etanercept and methotrexate therapy in rheumatoid arthritis. Ann Rheum Dis 73:2144–2151
Smolen JS, Nash P, Durez P et al (2013) Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (preserve): a randomised controlled trial. Lancet 381:918–929
van der Heijde D, Burmester G, Melo-Gomes J et al (2009) Inhibition of radiographic progression with combination etanercept and methotrexate in patients with moderately active rheumatoid arthritis previously treated with monotherapy. Ann Rheum Dis 68:1113–1118
van der Heijde D, Burmester G, Melo-Gomes J et al (2008) The safety and efficacy of adding etanercept to methotrexate or methotrexate to etanercept in moderately active rheumatoid arthritis patients previously treated with monotherapy. Ann Rheum Dis 67:182–188
van der Heijde D, Klareskog L, Landewé R et al (2007) Disease remission and sustained halting of radiographic progression with combination etanercept and methotrexate in patients with rheumatoid arthritis. Arthritis Rheum 56:3928–3939
van der Heijde D, Klareskog L, Rodriguez-Valverde V et al (2006) Comparison of etanercept and methotrexate, alone and combined, in the treatment of rheumatoid arthritis: two-year clinical and radiographic results from the tempo study, a double-blind, randomized trial. Arthritis Rheum 54:1063–1074
van der Heijde D, Klareskog L, Singh A et al (2006) Patient reported outcomes in a trial of combination therapy with etanercept and methotrexate for rheumatoid arthritis: the TEMPO trial. Ann Rheum Dis 65:328–334
van Riel PL, Taggart AJ, Sany J et al (2006) Efficacy and safety of combination etanercept and methotrexate versus etanercept alone in patients with rheumatoid arthritis with an inadequate response to methotrexate: the ADORE study. Ann Rheum Dis 65:1478–1483
Richter A, Listing J, Kekow J et al (2014) Biologic monotherapy: a treatment option for elderly RA patients with multimorbid conditions. Ann Rheum Dis 73:491–491
Emery P, Sebba A, Huizinga TW (2013) Biologic and oral disease-modifying antirheumatic drug monotherapy in rheumatoid arthritis. Ann Rheum Dis 72:1897–1904
Black N (1996) Why we need observational studies to evaluate the effectiveness of health care. BMJ 312:1215–1218
Strangfeld A, Richter A (2015) How do register data support clinical decision-making? Z Rheumatol 74:119–124
Lautenschlager J, Mau W, Kohlmann T et al (1997) Comparative evaluation of a German version of the health assessment questionnaire and the Hannover functional capacity questionnaire. Z Rheumatol 56:144–155
Gabay C, Hasler P, Kyburz D et al (2014) Biological agents in monotherapy for the treatment of rheumatoid arthritis. Swiss Med Wkly 144:w13950
Albrecht K, Krüger K, Wollenhaupt J et al (2014) German guidelines for the sequential medical treatment of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. Rheumatol Int 34:1–9
Acknowledgements
We thank the study investigators, the staff, and the patients for their participation in this trial. Editorial/medical writing support was provided by Neel Misra and Rina Vekaria Passmore of Engage Scientific Solutions and was funded by Pfizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the local Ethics Committee of the State Chamber of Physicians and of the University of Muenster, and all patients gave written informed consent. It was conducted in accordance with the Declaration of Helsinki and complied with local German regulations and German Drug Law.
Funding
This observational study was sponsored by Pfizer, which was responsible for collecting and analyzing the data.
Disclosures
Markus Gaubitz received speaker’s honoraria from AbbVie, Amgen, BMS, Boehringer Ingelheim, Chugai, Celgene, Grünenthal, Hexal, Janssen, Medac, MSD, Mundipharma, Pfizer, Roche, Servier, SOBI, and UCB.
Karl-Heinz Göttl declares that he has no conflicts of interest.
Olaf Behmer is an employee of Pfizer Pharma GmbH and has a financial interest in Pfizer.
Ralph Lippe is an employee of Pfizer Pharma GmbH and has a financial interest in Pfizer.
Thomas Meng is an employee of Pfizer Pharma GmbH and has a financial interest in Pfizer.
Peter-Andreas Löschmann is an employee of Pfizer Pharma GmbH and has a financial interest in Pfizer.
Additional information
Trial registration: ClinicalTrials.gov, NCT00488475
Rights and permissions
About this article
Cite this article
Gaubitz, M., Göttl, KH., Behmer, O. et al. Etanercept is effective as monotherapy or in combination with methotrexate in rheumatoid arthritis: subanalysis of an observational study. Clin Rheumatol 36, 1989–1996 (2017). https://doi.org/10.1007/s10067-017-3757-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3757-8