Abstract
Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis (RA). This post hoc analysis investigated the effect of methotrexate (MTX) dose on the efficacy of tofacitinib in patients with RA. ORAL Scan (NCT00847613) was a 2-year, randomized, Phase 3 trial evaluating tofacitinib in MTX-inadequate responder (IR) patients with RA. Patients received tofacitinib 5 or 10 mg twice daily (BID), or placebo, with low (≤12.5 mg/week), moderate (>12.5 to <17.5 mg/week), or high (≥17.5 mg/week) stable background MTX. Efficacy endpoints (at months 3 and 6) included American College of Rheumatology (ACR) 20/50/70 response rates, and mean change from baseline in Clinical Disease Activity Index (CDAI), Disease Activity Score in 28 joints (DAS28)–4(erythrocyte sedimentation rate [ESR]), Health Assessment Questionnaire-Disability Index (HAQ-DI), and modified Total Sharp score. 797 patients were treated with tofacitinib 5 mg BID (N = 321), tofacitinib 10 mg BID (N = 316), or placebo (N = 160); 242, 333, and 222 patients received low, moderate, and high MTX doses, respectively. At months 3 and 6, ACR20/50/70 response rates were greater for both tofacitinib doses vs placebo across all MTX doses. At month 3, mean changes from baseline in CDAI and HAQ-DI were significantly greater for both tofacitinib doses vs placebo, irrespective of MTX category; improvements were maintained at month 6. Both tofacitinib doses demonstrated improvements in DAS28–4(ESR), and less structural progression vs placebo, across MTX doses at month 6. Tofacitinib plus MTX showed greater clinical and radiographic efficacy than placebo in MTX-IR patients with RA, regardless of MTX dose.
Similar content being viewed by others
Introduction
Current guidelines advise that treatment of rheumatoid arthritis (RA) should be initiated with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), such as methotrexate (MTX). For patients with inadequate response (IR) to csDMARDs, options include biologic DMARDs (bDMARDs), such as tumor necrosis factor inhibitors (TNFi), or targeted synthetic small-molecule DMARDs (tsDMARDs), often given in combination with csDMARDs [1, 2].
Early intervention with csDMARDs, usually MTX, has been shown to be beneficial in achieving a good clinical response in a proportion of patients, with resulting modification of the course of the disease [1, 3, 4]. However, not all patients achieve remission with MTX. There is extensive evidence to suggest that concomitant use of a bDMARD with MTX is beneficial in achieving treatment goals [5–12]. However, it is not clear whether there is a minimum or maximum dose of MTX that, when given in combination with bDMARDs, can affect clinical outcomes. Recently, a study has shown that increasing doses of MTX administered concomitantly with adalimumab broadly associated with an increasing proportion of patients achieving clinical efficacy endpoints [13, 14]. A recent study in Japan has also shown that adalimumab plus ≥10 mg MTX consistently resulted in better improvement in disease activity score in 28 joints (DAS28) and more patients with DAS28-defined remission compared with adalimumab plus MTX <10 mg [15].
Tofacitinib is an oral Janus kinase inhibitor for the treatment of RA. The efficacy and safety of tofacitinib 5 and 10 mg twice daily (BID), as monotherapy or in combination with csDMARDs, have been demonstrated in Phase 2 and Phase 3 clinical trials and in long-term extension studies [16–27]. The tofacitinib clinical development program included both MTX-naïve patients and those with an IR to treatment with DMARDs, including csDMARDs (primarily MTX) and TNFi.
ORAL Scan (NCT00847613) was a Phase 3 clinical trial that investigated tofacitinib 5 and 10 mg BID vs placebo, all with stable background MTX, in MTX-IR patients with RA [24]. In ORAL Scan, tofacitinib plus MTX improved the signs and symptoms of RA, clinical disease activity, and reduced the progression of structural damage compared with placebo plus MTX at month 6. The structural damage results were statistically significant with tofacitinib 10 mg BID (p < 0.05), but not with tofacitinib 5 mg BID (p = 0.0792) [24].
This post hoc analysis of data from ORAL Scan investigated whether there was a difference in the benefit of tofacitinib when given with oral MTX at different dose ranges.
Materials and methods
Study design, patients, and MTX dose categories
ORAL Scan (NCT00847613) was a 2-year, multinational, randomized, double-blind, parallel-group, placebo-controlled Phase 3 clinical trial conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. The Institutional Review Board at each study center approved the protocol and all patients provided written informed consent.
Details of the study design and patient population have been reported previously [24]. Briefly, eligible patients were aged ≥18 years with active RA despite receiving MTX at a stable dose of 15–25 mg/week for ≥6 weeks (MTX doses <15 mg/week were permitted if there were safety issues at higher doses, but dose adjustments were not permitted during the study). Patients were randomized 4:4:1:1 to receive tofacitinib 5 mg BID, tofacitinib 10 mg BID, placebo advanced to tofacitinib 5 mg BID, or placebo advanced to tofacitinib 10 mg BID, all in combination with MTX. Placebo patients advanced to active treatment at month 3 if they did not meet a pre-defined response of ≥20 % improvement in swollen and tender joint counts. All remaining placebo patients mandatorily advanced to their assigned tofacitinib dose at month 6 regardless of clinical response.
In this post hoc analysis, patients were divided into three categories according to their baseline mean weekly MTX dose: low (≤12.5 mg/week), moderate (>12.5 to <17.5 mg/week), and high (≥17.5 mg/week). MTX doses were stable throughout the study as per protocol.
Efficacy assessments
Signs and symptoms and clinical disease activity were assessed at months 3 and 6 utilizing American College of Rheumatology 20, 50, and 70 % response rates (ACR20/50/70), least squares mean (LSM) change from baseline in Clinical Disease Activity Index (CDAI) score, and the proportion of patients achieving low disease activity (CDAI score ≤10) and remission (CDAI score ≤2.8). LSM change from baseline in DAS28–4(erythrocyte sedimentation rate [ESR]) was assessed at month 6.
Functional status was assessed by LSM change from baseline in the Health Assessment Questionnaire-Disability Index (HAQ-DI) score, including the proportion of patients achieving HAQ-DI <0.5.
Radiographic progression was assessed via LSM change from baseline in van der Heijde modified Total Sharp Score (mTSS), and the proportion of patients with no radiographic progression, defined as ≤0.5-unit increase from baseline in mTSS at month 6.
Statistical analyses
In this exploratory, post hoc analysis, the primary analysis population was the Full Analysis Set, which included all randomized patients who received ≥1 dose of study medication and had ≥1 post-baseline measurement. To handle missing data, no imputation was applied to descriptive statistics of patient demographics and baseline characteristics. For binary efficacy endpoints, non-responder imputation (NRI) was applied. In particular, patients who withdrew from a study for any reason before month 6, or patients who advanced treatment to active tofacitinib after month 3, had their values on or after withdrawing or advancing treatment set to “non-responder” in the efficacy analyses. Longitudinal continuous variables were analyzed using a mixed-effect repeated measure model with no imputation for missing data. The fixed effects of treatment, visit, and treatment-by-visit interaction were included, with subject as a random effect.
This was a post hoc analysis, and no adjustment was made for multiple testing. For the efficacy analyses, 95 % confidence intervals (CIs) for each tofacitinib group vs placebo were calculated in each MTX dose category; a CI that did not contain 0 was taken to indicate that the difference between tofacitinib and placebo was significant. Logistic regression analyses for binary variables and linear regression analyses for continuous variables were used to evaluate the effect of various baseline factors (CDAI, DAS28–4[ESR], HAQ-DI, body mass index [BMI], glucocorticoid [GC] use, MTX dose, swollen joints, and tender joints) on efficacy responses. A multivariate model was developed that incorporated variables from a univariate model with p values <0.10 and removed factors using stepwise, backward, and forward methods until only significant factors remained (p < 0.05).
Results
Patients
A total of 797 patients were randomized to receive tofacitinib 5 mg BID (N = 321), tofacitinib 10 mg BID (N = 316), or placebo (N = 160), all in combination with background MTX; patients continued to receive concomitant MTX at the dose they were receiving at study start. The number of patients in the low, moderate, and high MTX dose categories was 242 (30 %), 333 (42 %), and 222 (28 %), respectively (Table 1). The mean weekly MTX dose in the three categories was 9.4, 15.0, and 20.9 mg/week, respectively (Table 1).
Baseline demographics and disease characteristics were generally similar across the MTX dose categories. BMI, proportion of Caucasian patients, GC use, swollen and tender joint counts, and CDAI scores tended to be higher among patients in the high MTX dose category, and proportion of patients with prior TNFi therapy, which tended to be higher in the low MTX dose category (Table 1).
Efficacy
Clinical and functional outcomes
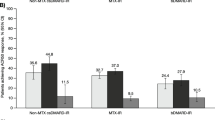
The proportion of patients achieving ACR20/50/70 response rates was significantly greater for those receiving tofacitinib 5 and 10 mg BID vs placebo, regardless of MTX dose level, at both month 3 and month 6. The only exception was ACR70 for tofacitinib 5 mg BID at month 3 in the moderate MTX dose group, which was numerically greater than placebo (Fig. 1).
Tofacitinib 5 and 10 mg BID-treated patients achieved significantly greater reductions from baseline in CDAI scores at month 3, compared with placebo, irrespective of MTX dose (Fig. 2a). Other than the tofacitinib 5 mg BID vs placebo comparison in the high MTX dose group, significant improvements in CDAI scores (Fig. 2b) and DAS28–4(ESR) scores (Table 2) were maintained at month 6 in all three MTX dose categories.
Change from baseline in a CDAI at month 3, b CDAI at month 6, c HAQ-DI at month 3, and d HAQ-DI at month 6. *p < 0.05, **p < 0.001, ***p < 0.0001 vs placebo; Full Analysis Set. BID twice daily, CDAI Clinical Disease Activity Index, CFB change from baseline, HAQ-DI Health Assessment Questionnaire-Disability Index, LSM least squares mean, MTX methotrexate, N number of patients assessed, SE standard error, wk week
At month 6, significantly higher proportions of patients treated with both doses of tofacitinib achieved CDAI low disease activity (CDAI score ≤10) and remission (CDAI score ≤2.8) compared with the placebo group, regardless of background MTX dose, with the exception of the tofacitinib 5 mg group with low-dose MTX for CDAI remission (Table 2).
Improvements from baseline in HAQ-DI were significantly greater for tofacitinib 5 and 10 mg BID vs placebo in all MTX dose groups at months 3 and 6, other than tofacitinib 5 mg BID in the high-dose MTX group at month 6 (Fig. 2c, d). At month 6, significantly higher proportions of patients treated with tofacitinib 5 and 10 mg BID vs placebo in all MTX dose groups achieved HAQ-DI <0.5, except for tofacitinib 5 mg BID in the high-dose MTX group (Table 2).
Radiographic progression
No clear relationship between radiographic progression (as measured by LSM change from baseline in mTSS scores and the proportion of patients with no radiographic progression) and MTX dose was observed at month 6 with tofacitinib 5 or 10 mg BID (Fig. 3a, b). Generally, less radiographic progression was observed with both tofacitinib 5 and 10 mg BID plus MTX compared with placebo plus MTX (Fig. 3a, b). In the placebo-treated patients, less radiographic progression was demonstrated in the moderate and high MTX dose groups compared with the low MTX dose group (Fig. 3a).
Radiographic progression at month 6 by MTX dose category: a LSM change from baseline in mTSS and b proportion of patients with no radiographic progression (mTSS ≤0.5). *p < 0.05 vs placebo; aNon-responder imputation; Full Analysis Set. BID twice daily, CFB change from baseline, CI confidence interval, LSM least squares mean, mTSS modified Total Sharp/van der Heijde Score, MTX methotrexate, SE standard error, wk week
Efficacy analyses by MTX dose and baseline variables
The univariate and multivariate regression analyses performed to assess the effect of baseline variables on efficacy outcomes showed no significant effect of BMI, GC use, or MTX dose on disease activity assessments with either tofacitinib 5 or 10 mg BID (Supplementary Table 1 in Online Resource 1).
Discussion
It has been shown that the concomitant use of a bDMARD with MTX can be clinically beneficial in MTX-IR patients; what has not been demonstrated conclusively is whether there is a minimum dose of MTX that, when given in combination with bDMARDs, affects clinical outcomes. In a previous post hoc analysis of data from the tofacitinib clinical RA program, broadly similar efficacy was seen in studies with tofacitinib administered as monotherapy and tofacitinib given in combination with MTX [28]. Whereas the earlier analysis used data from four different clinical studies, this post hoc analysis of data from the Phase 3 ORAL Scan study was performed to establish whether the efficacy of tofacitinib 5 mg BID or 10 mg BID is affected by the dose of concomitant MTX within a single study. The MTX-IR population for this post hoc analysis was similar to MTX-IR patients with RA who are candidates for tofacitinib or bDMARDs in clinical practice. The finding of whether there is a dose-dependent effect of concomitant MTX on clinical efficacy with tofacitinib is therefore clinically relevant.
Analysis of data from the ORAL Scan study revealed that both tofacitinib 5 and 10 mg BID were more effective in improving clinical activity and functional status in RA patients compared with placebo, regardless of the background MTX dose. The proportion of patients achieving ACR20/50/70 response rates, CDAI LDA ≤10, CDAI remission ≤2.8, and HAQ-DI <0.5 from baseline were greater for both tofacitinib doses compared with placebo, regardless of MTX dose level. Improvements from baseline in HAQ-DI, CDAI, and DAS28–4(ESR) were greater for both tofacitinib doses plus MTX compared with placebo plus MTX, regardless of MTX dose level.
Generally, less radiographic progression was observed at month 6 with both tofacitinib doses plus MTX compared with placebo plus MTX; the inhibition of radiographic progression at month 6 with either dose of tofacitinib did not appear to be MTX dose-dependent. In this post hoc analysis, tofacitinib 5 mg BID plus MTX showed significantly lower radiographic progression vs placebo plus MTX at month 6 in the low and high MTX dose groups, but a significant difference was not observed in the overall analysis [24]. In general, as expected, at month 6, higher doses of MTX in the placebo-treated patients with background MTX achieved improved clinical response and improved functional status with less radiographic progression than the lowest dose of MTX.
Concomitant use of MTX with all currently approved bDMARDs has consistently been associated with better efficacy than bDMARD monotherapy, but studies examining the effect of MTX dose on the efficacy of bDMARDs have reported differing findings. In a trial of MTX- and bDMARD-naïve patients treated with adalimumab 40 mg every other week in combination with MTX (2.5, 5, 10, or 20 mg per week), a trend of increased efficacy with increasing MTX dose was noted; however, the efficacy of adalimumab plus 10 or 20 mg of MTX weekly appeared equivalent [13]. Similar results were reported in the Dutch RhEumatoid Arthritis Monitoring (DREAM) registry [14]. In this report, there did not appear to be a difference in efficacy of adalimumab, etanercept, or infliximab in patients receiving 10, 15, 20, or 25 mg/week concomitant MTX. In the MUSICA trial, in which patients were treated with adalimumab 40 mg every other week in combination with low- or high-dose MTX (7.5 or 20 mg per week), small differences in terms of efficacy were observed between the two MTX groups but higher responses and some significant differences were reported with high-dose MTX [29]. In another study, a sub-analysis of pooled Phase 3 data of certolizumab pegol revealed no difference in clinical effect with different MTX doses [30].
It has been postulated that the mechanism for the increased efficacy of bDMARDs, especially monoclonal antibodies, with concomitant MTX vs bDMARD monotherapy is the impact of MTX on serum levels of bDMARDs. A study on the concentration-effect curve of adalimumab demonstrated that concomitant administration of MTX was associated with significantly higher trough levels of adalimumab compared with adalimumab monotherapy; the median level with concomitant MTX use was within the range of adalimumab concentrations thought necessary for clinical efficacy [31]. One mechanism of clearance of adalimumab from serum is related to the production of antidrug antibodies (ADAb) [31], with patients producing higher amounts of ADAb having reduced serum concentrations of functional adalimumab [32]. MTX, an immunomodulator, has been shown to reduce the immunogenicity of adalimumab in a dose-dependent manner [33]. An investigation of the co-administration of MTX 15–25 mg/week with tofacitinib in patients with RA reported no clinically significant effect on the pharmacokinetic profile of either drug [34].
Limitations of this post hoc analysis include low patient numbers in some treatment groups. The analysis could not determine definitively whether there is a differential effect of tofacitinib plus MTX depending on MTX dose. There were also some differences between groups in baseline characteristics, although these did not appear to affect efficacy assessments and interpretation. In the present analysis, patients had to have active disease despite treatment with MTX for inclusion in the study and therefore had limited response to MTX, irrespective of the dose. However, the majority of patients responded when tofacitinib was added to MTX. To determine the effect of MTX dose on the efficacy of tofacitinib, a study that includes csDMARD-, bDMARD-, and ts-DMARD-naïve patients assigned to varying doses of MTX plus concomitant tofacitinib or MTX monotherapy is required, similar to the study conducted by Burmester and colleagues for adalimumab [13]. Such a study could show unequivocally whether there is a dose response of MTX on tofacitinib efficacy, together with information on any differences in safety with increasing MTX dose.
In conclusion, this post hoc analysis confirms the main findings from ORAL Scan. Tofacitinib plus MTX generally showed greater clinical and radiographic efficacy than placebo plus MTX in MTX-IR patients with RA. The findings reported here suggest that in this population, the effect of tofacitinib is irrespective of background MTX dose, and that concomitant high-dose MTX may not be needed for tofacitinib efficacy.
References
Singh JA, Saag KG, Bridges SL Jr et al (2016) 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68:1–26
Smolen JS, Landewé R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73:492–509
Anderson JJ, Wells G, Verhoeven AC, Felson DT (2000) Factors predicting response to treatment in rheumatoid arthritis: the importance of disease duration. Arthritis Rheum 43:22–29
Smolen JS, Breedveld FC, Burmester GR et al (2016) Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis 75:3–15
Maini R, St Clair EW, Breedveld F, Furst D, Kalden J, Weisman M, Smolen J, Emery P, Harriman G, Feldmann M, Lipsky P (1999) Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT study group. Lancet 354:1932–1939
Weinblatt ME, Kremer JM, Bankhurst AD, Bulpitt KJ, Fleischmann RM, Fox RI, Jackson CG, Lange M, Burge DJ (1999) A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. Engl J Med 340:253–259
Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK (2003) Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 48:35–45
Keystone E, Heijde Dv, Mason D Jr, Landewe R, Vollenhoven RV, Combe B, Emery P, Strand V, Mease P, Desai C, Pavelka K (2008) Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum 58:3319–3329
Keystone EC, Genovese MC, Klareskog L, Hsia EC, Hall ST, Miranda PC, Pazdur J, Bae SC, Palmer W, Zrubek J, Wiekowski M, Visvanathan S, Wu Z, Rahman MU (2009) Golimumab, a human antibody to tumour necrosis factor {alpha} given by monthly subcutaneous injections, in active rheumatoid arthritis despite methotrexate therapy: the GO-FORWARD study. Ann Rheum Dis 68:789–796
Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, Chen A, Armstrong GK, Douglass W, Tyrrell H (2010) Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab’s Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis 69:1629–1635
Emery P, Burmester GR, Bykerk VP, Combe BG, Furst DE, Barre E, Karyekar CS, Wong DA, Huizinga TW (2015) Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 74:19–26
Burmester GR, Rigby WF, van Vollenhoven RF, Kay J, Rubbert-Roth A, Kelman A, Dimonaco S, Mitchell N (2016) Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis 75:1081–1091
Burmester GR, Kivitz AJ, Kupper H, Arulmani U, Florentinus S, Goss SL, Rathmann SS, Fleischmann RM (2015) Efficacy and safety of ascending methotrexate dose in combination with adalimumab: the randomised CONCERTO trial. Ann Rheum Dis 74:1037–1044
Manders SH, Kievit W, Adang E, Jansen TJ, Stolk JN, Visser H, Schilder AM, Vonkeman HE, van de Laar MA, van Riel PL (2015) Effectiveness of TNF inhibitor treatment with various methotrexate doses in patients with rheumatoid arthritis: results from clinical practice. Ann Rheum Dis 74:e24
Nakashima Y, Miyahara H, Kondo M, Fukuda T, Harada H, Haraguchi A, Inoue Y, Ishinishi T, Maekawa M, Maeyama A, Nakashima M, Shono E, Suematsu E, Shimauchi T, Tsuru T, Tsukamoto H, Yoshizawa S, Yoshizawa S, Iwamoto Y (2016) Impact of methotrexate dose on efficacy of adalimumab in Japanese patients with rheumatoid arthritis: results from registered data analyses. Mod Rheumatol:1–7
Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, Krishnaswami S, Burgos-Vargas R, Wilkinson B, Zerbini CAF, Zwillich SH (2009) The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum 60:1895–1905
Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH, Tofacitinib Study Investigators (2011) Phase II study of tofacitinib (CP-690,550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res (Hoboken) 63:1150–1158
Fleischmann R, Cutolo M, Genovese MC, Lee EB, Kanik KS, Sadis S, Connell CA, Gruben D, Krishnaswami S, Wallenstein G, Wilkinson BE, Zwillich SH (2012) Phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) or adalimumab monotherapy versus placebo in patients with active rheumatoid arthritis with an inadequate response to disease-modifying antirheumatic drugs. Arthritis Rheum 64:617–629
Kremer JM, Cohen S, Wilkinson BE, Connell CA, French JL, Gomez-Reino J, Gruben D, Kanik KS, Krishnaswami S, Pascual-Ramos V, Wallenstein G, Zwillich SH (2012) A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 64:970–981
Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S (2015) Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: a 12-week, randomized, phase 2 study. Mod Rheumatol 25:514–521
van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, García Meijide JA, Wagner S, Forejtova S, Zwillich SH, Gruben D, Koncz T, Wallenstein GV, Krishnaswami S, Bradley JD, Wilkinson B, ORAL Standard Investigators (2012) Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med 367:508–519
Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, Gruben D, Wallenstein GV, Zwillich SH, Kanik KS, ORAL Solo Investigators (2012) Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 367:495–507
Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Soma K, Bradley J, Mebus C, ORAL Step investigators (2013) Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 381:451–460
van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, Cardiel MH, Cohen S, Nash P, Song YW, Tegzová D, Wyman BT, Gruben D, Benda B, Wallenstein G, Krishnaswami S, Zwillich SH, Bradley JD, Connell CA, ORAL Scan Investigators (2013) Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum 65:559–570
Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, Isaacs JD, Gruben D, Wallenstein G, Krishnaswami S, Zwillich SH, Koncz T, Riese R, Bradley J (2013) Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med 159:253–261
Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley J, Gruben D, Koncz T, Krishnaswami S, Wallenstein G, Zang C, Zwillich S, van Vollenhoven R, on behalf of the ORAL Start investigators (2014) Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med 370:2377–2386
Wollenhaupt J, Silverfield J, Lee EB, Curtis JR, Wood SP, Soma K, Nduaka CI, Benda B, Gruben D, Nakamura H, Komuro Y, Zwillich SH, Wang L, Riese RJ (2014) Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, for the treatment of rheumatoid arthritis in open-label, longterm extension studies. J Rheumatol 41:837–852. doi:53420
Keystone E, Fleischmann R, van Vollenhoven R, Kremer J, Gruben D, Bradley J, Riese R, Mebus C, Wallenstein G, Zwillich SH, Benda B, Krishnaswami S (2013) Tofacitinib, an oral Janus kinase inhibitor: post-hoc analyses of efficacy and safety of monotherapy versus combination therapy in a phase 3 rheumatoid arthritis population. Ann Rheum Dis 72:242
Kaeley GS, Evangelisto AM, Nishio MJ, Goss SL, Liu S, Kalabic J, Kupper H (2016) Methotrexate Dosage Reduction Upon Adalimumab Initiation: Clinical and Ultrasonographic Outcomes from the Randomized Noninferiority MUSICA Trial. J Rheumatol Epub ahead of print
Combe B, Furst DE, Keystone EC, van der Heijde D, Luijtens K, Ionescu L, Goel N, Emery P (2016) Certolizumab pegol efficacy across methotrexate regimens: a pre-specified analysis of two phase III trials. Arthritis Care Res (Hoboken) 68:299–307
Pouw MF, Krieckaert CL, Nurmohamed MT, van der Kleij D, Aarden L, Rispens T, Wolbink G (2015) Key findings towards optimising adalimumab treatment: the concentration-effect curve. Ann Rheum Dis 74:513–518
van Schouwenburg PA, van de Stadt LA, de Jong RN, van Buren EE, Kruithof S, de Groot E, Hart M, van Ham SM, Rispens T, Aarden L, Wolbink GJ, Wouters D (2013) Adalimumab elicits a restricted anti-idiotypic antibody response in autoimmune patients resulting in functional neutralisation. Ann Rheum Dis 72:104–109
Krieckaert CL, Nurmohamed MT, Wolbink GJ (2012) Methotrexate reduces immunogenicity in adalimumab treated rheumatoid arthritis patients in a dose dependent manner. Ann Rheum Dis 71:1914–1915
Cohen S, Zwillich SH, Chow V, LaBadie RR, Wilkinson B (2010) Co-administration of the JAK inhibitor CP-690,550 and methotrexate is well tolerated in patients with rheumatoid arthritis without need for dose adjustment. Br J Clin Pharmacol 69:143–151
Acknowledgments
We wish to thank all patients who participated in this trial and investigators and staff of the participating centers. This study was funded by Pfizer Inc. Medical writing support was provided by Carole Evans, PhD, on behalf of Complete Medical Communications and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest disclosures
R Fleischmann has received grants and/or research support from, and has acted as a consultant for, Pfizer Inc.
PJ Mease has participated in speakers’ bureaus and/or received grants and/or research support from AbbVie, Amgen, Biogen, Bristol Myers Squibb, Celgene, Crescendo, Genentech, Janssen, Lilly, Merck, Novartis, Pfizer Inc, UCB, and Vertex.
S Schwartzman has participated as a speaker and as a consultant for Pfizer Inc.
L-J Hwang, K Soma, C Connell, L Takiya, and E Bananis are employees and shareholders of Pfizer Inc.
Disclosure
This study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonisation Guidelines for Good Clinical Practice. The Institutional Review Board at each study center approved the protocol and all patients provided written informed consent.
Electronic supplementary material
ESM 1
(DOC 95 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Fleischmann, R., Mease, P., Schwartzman, S. et al. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by background methotrexate dose group. Clin Rheumatol 36, 15–24 (2017). https://doi.org/10.1007/s10067-016-3436-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3436-1