Abstract
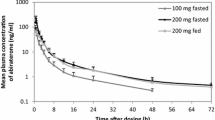
SoluMatrix® meloxicam has been developed using SoluMatrix Fine Particle Technology™ to produce a meloxicam drug product with enhanced absorption properties to enable treatment at lower doses than available oral meloxicam drug products. This follows recognition of serious dose-dependent adverse events (AEs) associated with nonsteroidal anti-inflammatory drugs, including meloxicam. This study investigated the pharmacokinetic (PK) properties of SoluMatrix meloxicam 5-mg (fasting conditions) and 10-mg capsules (fasting and fed conditions) and compared SoluMatrix meloxicam 10-mg capsules with meloxicam 15-mg tablets under fasting conditions. This four-period crossover study randomized 28 healthy adult participants to receive single doses of SoluMatrix meloxicam 5-mg capsules (fasting) and 10-mg capsules (fasting or fed) and meloxicam tablets 15 mg (fasting). Meloxicam plasma concentrations were assessed through 96 h postdose. Safety was assessed. Twenty-five participants (89.3 %) completed the study. Under fasting conditions, SoluMatrix meloxicam 10 mg [1252.8 (254.22) ng/mL] produced similar meloxicam mean (standard deviation (SD)) maximum plasma concentrations vs meloxicam 15-mg tablets [1288.8 (424.40) ng/mL]. The overall mean (SD) systemic meloxicam exposure was 33 % lower for SoluMatrix meloxicam 10 mg [29,173.01 (11,042.09) ng*h/mL] vs meloxicam 15-mg tablets [40,875.6 (11,733.47) ng*h/mL]. The median time to maximum plasma meloxicam levels occurred earlier following SoluMatrix meloxicam 5 mg (2.0 h) and 10 mg (2.0 h) administration vs meloxicam 15-mg tablets (4.0 h). Few study-medication-related AEs were reported. SoluMatrix meloxicam 10 mg was more rapidly absorbed and associated with a lower overall exposure compared with meloxicam 15-mg tablets in this study in healthy adults under fasting conditions.

Similar content being viewed by others
Notes
SoluMatrix® is a registered trademark of iCeutica Pty Ltd and is licensed to Iroko.
SoluMatrix Fine Particle Technology ™ is a trademark of iCeutica Inc., and the technology is licensed to Iroko for exclusive use in NSAIDs.
References
Full prescribing information for Mobic. (2011) Boehringer Ingelheim International GmbH http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020938s022lbl.pdf. Accessed 23 April 2015
Bartholow M (2013) Top 200 Drugs of 2012. Intellisphere, LLC. http://www.pharmacytimes.com/publications/issue/2013/July2013/Top-200-Drugs-of-2012. Accessed 24 April 2015
IMS National prescription audit. (2015) IMS Health. imshealth.com.
Garcia Rodriguez LA, Barreales Tolosa L (2007) Risk of upper gastrointestinal complications among users of traditional NSAIDs and COXIBs in the general population. Gastroenterology 132(2):498–506. doi:10.1053/j.gastro.2006.12.007
McGettigan P, Henry D (2011) Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med 8(9):e1001098. doi:10.1371/journal.pmed.1001098
Asghar W, Jamali F (2015)The effect of COX-2-selective meloxicam on the myocardial, vascular and renal risks: a systematic review. Inflammopharmacol 23(1):1–16
Food and Drug Administration. Public Health Advisory (2005) FDA announces important changes and additional warnings for COX-2 selective and non-selective non-steroidal anti-inflammatory drugs (NSAIDs). Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm150314.htm. Accessed 11 November 2015
Center for Drug Evaluation and Research (CDER) FaDA (2002) Guidance for industry: food-effect bioavailability and fed bioequivalence studies. US department of health and human services. http://www.fda.gov/downloads/regulatoryinformation/guidances/ucm126833.pdf. Accessed 7 May 2015
ICH Expert Working Group (1996) ICH harmonised tripartite guideline: guideline for good clinical practice e6(r1). International Conference on Harmonisation. http://www.ich.org/fileadmin/ Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed 7 May 2015
WMADeclaration ofHelsinki (2015) Ethical principles formedical research involving human subjects. http://www.wma.net/en/ 30publications/10policies/b3/. Accessed 8 June 2015
Yocum D, Fleischmann R, Dalgin P, Caldwell J, Hall D, Roszko P (2000) Safety and efficacy of meloxicam in the treatment of osteoarthritis: a 12-week, double-blind, multiple-dose, placebo-controlled trial. The Meloxicam Osteoarthritis Investigators. Arch Intern Med 160(19):2947–2954
Hosie J, Distel M, Bluhmki E (1996) Meloxicam in osteoarthritis: a 6-month, double-blind comparison with diclofenac sodium. Br J Rheumatol 35(Suppl 1):39–43
Valat JP, Accardo S, Reginster JY, Wouters M, Hettich M, Lieu PL, International Meloxicam Lumbar Osteoarthritis Group (2001) A comparison of the efficacy and tolerability of meloxicam and diclofenac in the treatment of patients with osteoarthritis of the lumbar spine. Inflamm Res: Off J Eur Histam Res Soc 50(Suppl 1):S30–34
Busch U, Heinzel G, Narjes H (1991) Effect of food on pharmacokinetics of meloxicam, a new non steroidal anti-inflammatory drug (NSAID). Agents Actions 32(1–2):52–53
Türck D, Busch U, Heinzel G, Narjes H, Nehmiz G (1995) Effect of food on the pharmacokinetics of meloxicam after oral administration. Clin Drug Investig 9(5):270–276
Malec M, Shega JW (2015) Pain management in the elderly. Med Clin North Am 99(2):337–350. doi:10.1016/j.mcna.2014.11.007
Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, Taylor RS (2008) Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess 12(11):1–278
Argoff CE (2011) Recent developments in the treatment of osteoarthritis with NSAIDs. Curr Med Res Opin 27(7):1315–1327. doi:10.1185/03007995.2011.568058
Altman R, Hochberg M, Gibofsky A, Jaros M, Young C (2015) Efficacy and safety of low-dose soluMatrix meloxicam in the treatment of osteoarthritis pain: A 12-week, phase 3 study. Curr Med Res Opin:1–37. doi:10.1185/03007995.2015.1112772
Iroko Pharmaceuticals LLC (2013) Study of meloxicam capsules in subjects with osteoarthritis of the knee or hip. U.S. National Institutes of Health. https://clinicaltrials.gov/ct2/show/NCT01801735?term=SoluMatrix+meloxicam&rank=2. Accessed 10 May 2015
Full Prescribing Information for VIVLODEX. Iroko Pharmaceuticals, LLC (2015). Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207233s000lbl.pdf. Accessed 17 November 2015
Acknowledgments
This study was sponsored by Iroko Pharmaceuticals, LLC. Editorial assistance from Jill See, PhD; and Cole Brown, MD; of AlphaBioCom, LLC, was funded by Iroko. The authors thank Alexis Gomez, Olaolu Imasogie, Claire Sheridan, Melanie Lauterio, Jason Ferrante, and the participants and investigators who participated in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
PAREXEL International Corporation (Baltimore, MD, USA) conducted the study and analyses. The study was approved by the Aspire Institutional Review Board (Santee, CA, USA) and conducted in accordance with the Declaration of Helsinki, including the International Conference on Harmonisation principles of Good Clinical Practice [9, 10]. Participants provided written informed consent prior to study conduct.
Conflict of interest
Azra Hussaini is an employee of PAREXEL International Corporation, Baltimore, MD, USA. Daniel Solorio and Clarence Young are employees of Iroko Pharmaceuticals, LLC, Philadelphia, PA, USA.
Rights and permissions
About this article
Cite this article
Hussaini, A., Solorio, D. & Young, C. Pharmacokinetic properties of low-dose SoluMatrix meloxicam in healthy adults. Clin Rheumatol 35, 1099–1104 (2016). https://doi.org/10.1007/s10067-015-3121-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3121-9