Abstract
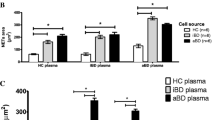
Increased neutrophil chemotaxis and hyperresponsiveness to streptococci have been considered to play a role in Behçet’s disease (BD) pathogenesis. Our aim was to correlate TLR2 expression and chemotactic responses stimulated by bacterial lipoteichoic acid (LTA) in BD neutrophils. Thus, we assessed expressions of TLR2 and the correlate receptors CD14, CD114 (G-CSF receptor), CD116 (GM-CSF receptor) and also TLR4 on circulating neutrophils and monocytes of patients with active BD. Serum concentration of soluble CD14 (sCD14) was also measured. Neutrophil chemotactic responses from BD patients and healthy controls under LTA stimulation were assessed. Disease activity was evaluated by Behçet’s Disease Current Activity Form (BDCAF). Receptor expressions were measured by flow cytometry, neutrophil chemotaxis was assessed in a Boyden chamber and sCD14 was measured by enzyme-linked immunosorbent assay. TLR2 expression was higher only on BD monocytes compared with healthy controls (39.9 ± 13.1 vs. 33.6 ± 5.3, p = 0.019). Expressions of all other receptors were similar in BD and control group. Of particular interest, TLR2 expression on neutrophils was similar in both groups and, when stimulated with LTA, BD neutrophils showed chemotactic responses similar to the controls. Neutrophils exhibited increased chemotaxis only when incubated with BD plasma. Serum sCD14 concentration was higher in BD patients than in controls (1,920.8 ± 563.6 vs. 1,623.2 ± 391.3 ng/ml, p = 0.008), and it was positively correlated with both CD14 expression on circulating monocytes membrane (p = 0.035) and BDCAF scores (p = 0.025). In conclusion, isolated BD neutrophils do not overexpress TLR2 neither overreact to LTA. However, because TLR2 expression was higher on BD monocytes, sCD14 from monocyte origin correlated with disease activity and neutrophil hyperchemotaxis occurred on strict dependence of plasmatic soluble factors, we suggest a possible role for bacterial stimulation of monocytes via TLR2 producing neutrophil-stimulating pro-inflammatory factors in BD.




Similar content being viewed by others
References
Yurdakul S, Hamuryudan V, Yazici H (2004) Behçet syndrome. Curr Opin Rheumatol 16:38–42
Direskeneli H (2006) Autoimmunity vs autoinflammation in Behçet’s disease: do we oversimplify a complex disorder? Rheumatology 45:1461–1465
Matsumara N, Mizushima Y (1975) Leucocyte movement and colchicine treatment in Behçet’s disease. Lancet 2:813
Carletto A, Pacor ML, Biasi D et al (1997) Changes of neutrophil migration without modification of in vitro metabolism and adhesion in Behçet’s disease. J Rheumatol 24:1332–1336
Tüzün B, Tüzün Y, Yurdakul S, Hamuryudan V, Yazici H, Ozyazgan Y (1999) Neutrophil chemotaxis in Behçet’s syndrome. Ann Rheum Dis 58:658
The Behçet’s Disease Research Committee of Japan (1989) Skin hypersensitivity to streptococcal antigens and the induction of systemic symptoms by the antigens in Behçet’s disease—a multicenter study. J Rheumatol 16:506–511
Lehner T (1999) Immunopathogenesis of Behçet’s disease. Ann Med Interne (Paris) 150:483–487
Direskeneli H, Eksioglu-Demiralp E, Yavuz S et al (2000) T cell responses to 60/65 kDa heat shock protein derived peptides in Turkish patients with Behçet’s disease. J Rheumatol 27:708–713
Calguneri M, Kiraz S, Ertenli I, Benekli M, Karaarslan Y, Celik I (1996) The effect of prophylactic penicillin treatment on the course of arthritis episodes in patients with Behçet’s disease. Arthritis Rheum 39:2062–2065
Niwa Y, Mizushima Y (1990) Neutrophil-potentiating factors released from stimulated lymphocytes; special reference to the increased in neutrophil-potentiating factors from streptococcus-stimulated lymphocytes of patients with Behçet’s disease. Clin Exp Immunol 79:353–360
Medzhitov R, Hurlburt-Preston P, Janeway CA Jr (1997) A human homologue of the Drosophila Toll protein signs activation of adaptive immunity. Nature 388:394–397
Roelofs MF, Abdollahi-Roodsaz S, Joosten LAB, van den Berg WB, Radstake TRDJ (2008) The orchestra of Toll-like receptor and their potential role in frequently occurring rheumatic conditions. Arthritis Rheum 58:338–348
Lotz S, Aga E, Wilde I et al (2004) Highly purified lipoteichoic acid activates neutrophil granulocytes and delays their spontaneous apoptosis via CD14 and TLR2. J Leuk Biol 75:467–477
Schröder NWJ, Morath S, Alexander C et al (2003) Lipoteichoic Acid (LTA) of streptococcus pneumoniae and staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lypopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 278:15587–15594
Kurt-Jones EA, Mandell L, Whitney C et al (2002) Role of Toll-like receptor 2 (TLR2) in neutrophil activation: GM-CSF enhances TLR2 expression and TLR-2 mediated interleukin 8 responses in neutrophils. Blood 100:1860–1868
Do JE, Kwon SY, Park S, Lee ES (2008) Effects of vitamin D on expression of Toll-like receptors of monocytes from patients with Behçet’s disease. Rheumatology 47:840–848
Kirino Y, Takeno M, Watanabe R et al (2008) Association of reduced heme oxygenase-1 with excessive Toll-like receptor 4 expression in peripheral blood mononuclear cells in Behçet’s disease. Arthritis Res Ther 10:R16
Nara K, Kurokawa MS, Chiba S et al (2008) Involvement of innate immunity in the pathogenesis of intestinal Behçet’s disease. Clin Exp Immunol 152:245–251
Yavuz S, Elbir Y, Tulunay A, Eksioglu-Demiralp E, Direskeneli H (2008) Differential expression of toll-like receptor 6 on granulocytes and monocytes implicates the role of microorganisms in Behçet’s disease etiopathogenesis. Rheumatol Int 28:401–406
Sabroe I, Prince LR, Jones EC et al (2003) Selective roles for Toll-like receptor (TLR)2 and TLR4 in the regulation of neutrophil activation and life span. J Immunol 170:5268–5275
Sabroe I, Jones EC, Whyte MKB, Dower SK (2005) Regulation of human neutrophil chemokine receptor expression and function by activation of Toll-like receptors 2 and 4. Immunology 115:90–98
Aomatsu K, Kato T, Fujita H et al (2007) Toll-like receptor agonists stimulate human neutrophil migration via activation of mitogen-activated protein kinases. Immunology 123:171–180
Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C (2004) CD14 is an acute-phase protein. J Immunol 172:4470–4479
International Study Group for Behçet’s Disease (1991) Criteria for diagnosis of Behçet’s Disease. Lancet 335:1078–1080
Neves FS, Moraes JCB, Kowalski SC, Goldenstein-Schainberg C, Lage LV, Gonçalves CR (2007) Cross-cultural adaptation of the Behçet’s Disease Current Activity Form (BDCAF) to Brazilian Portuguese language. Clin Rheumatol 26:1263–1267
Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ (1999) Behçet’s disease: evaluation of a new instrument to measure clinical activity. Rheumatology 38:728–733
Mumcu G, Inanc N, Yavuz S, Direskeneli H (2007) The role of infectious agents in the pathogenesis, clinical manifestations and treatment strategies in Behçet’s disease. Clin Exp Rheumatol 25(4 Suppl 45):S27–S33
Tunc R, Keyman E, Melikoglu M et al (2002) Target organ associations in Turkish patients with Behçet’s disease: a cross sectional study by exploratory factor analysis. J Rheumatol 29:2393–2396
Diri E, Mat C, Hamuryudan V, Yurdakul S, Hizli N, Yazici H (2001) Papulopustular skin lesions are seen more frequently in patients with Behçet’s syndrome who have arthritis: a controlled and masked study. Ann Rheum Dis 60:1074–1076
Sobel JD, Haim S, Obedeanu N, Meshulam T, Merzbach D (1977) Polymorphonuclear leukocyte function in Behçet’s disease. J Clin Pathol 30:250–253
Kurihara A, Ojima F, Tsurufuji S (1984) Analysis of the effect of an anti-inflammatory steroid, dexamethasone, on neutrophil chemotaxis in the Boyden chamber with a modified 51Cr-labeling method. J Pharmacobiodyn 7:747–754
Mege JL, Dilsen N, Sanguedolce V et al (1993) Overproduction of monocyte derived tumor necrosis factor alpha, interleukin (IL) 6, IL-8 and increase neutrophil superoxide generation in Behçet’s disease. A comparative study with familial Mediterranen fever and healthy subjects. J Rheumatol 20:1544–1549
Cuchacovich M, Merino G, Yamamoto JH et al (2005) Behçet’s disease patients present high levels of deglycosylated anti-lipoteichoic acid IgG and high IL-8 production after lipoteichoic acid stimulation. Clin Exp Rheumatol 23(4 Suppl.38):S27–S34
Sahin S, Lawrence R, Direskeneli H, Hamuryudan V, Yazici H, Akoglu T (1996) Monocyte activity in Behçet’s disease. Br J Rheumatol 35:424–429
Maliszewski CR (1991) CD14 and immune response to lipopolysaccharide. Science 252:1321–1322
Pugin J, Schürer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, Tobias PS (1993) Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci USA 90:2744–2748
Troelstra A, Giepmans BN, Van Kessel KP, Lichenstein HS, Verhoef J, Van Strijp JA (1997) Dual effects of soluble CD14 on LPS priming of neutrophils. J Leukoc Biol 61:173–178
Soler-Rodriguez AM, Zhang H, Lichenstein HS et al (2000) Neutrophil activation by bacterial lipoprotein versus lipopolysaccharide: differential requirements for serum and CD14. J Immunol 164:2674–2683
Acknowledgements
We are grateful to Maria Aurora Gomes da Silva and Maria de Fátima de Almeida for their contributions to our laboratory chemotaxis experiments and to Rogério Ruscitto do Prado for statistical assistance. Discussions with many colleagues were important during this study, but we are particularly grateful to Dr. Roger Chammas, Ph.D. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [Grant number 2007/52448-4].
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Neves, F.S., Carrasco, S., Goldenstein-Schainberg, C. et al. Neutrophil hyperchemotaxis in Behçet’s disease: a possible role for monocytes orchestrating bacterial-induced innate immune responses. Clin Rheumatol 28, 1403–1410 (2009). https://doi.org/10.1007/s10067-009-1261-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-009-1261-5