Abstract
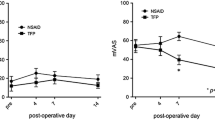
Current treatment guidelines advocate opioids for arthritis when standard analgesics produce inadequate relief. Efficacy, adverse effects (AEs), dosing regimens, physician expertise and patient preference influence treatment selection. This study assessed transdermal fentanyl (TDF) as a treatment option for osteoarthritis (OA) patients. This prospective, Canadian open-label, 8-week trial assessed the efficacy and safety of TDF in patients with OA of hip or knee with moderate-to-severe target joint pain inadequately controlled using weak opioids. TDF was initiated at 25 mcg/h and titrated to optimal pain control. Rescue acetaminophen 500 mg was allowed (maximum 4 g/day). The main endpoint was improvement in pain control assessment rating (five rating categories); pain intensity (0–10 numerical scale), functionality (WOMAC-OA Index), health-related quality of life (SF-36 Health Survey) and global impression were also evaluated. Eighty-one patients (61% female, mean age 60 years) were enrolled; 62 were evaluable. All had failed on previous weak opioid therapy, primarily codeine or codeine combinations. At treatment end, 65% rated pain control as improved (Pain Control Assessment rating change ≥1 category; p < 0.0001); mean change in pain intensity was a reduction of greater than 2 (p < 0.0001); almost 50% were maintained on TDF 25 mcg/h with less than 1.3 g/day of rescue acetaminophen. At 1 month and end of treatment, changes in the SF-36 physical global scale and individual sub-scores for the pain index and role-physical scales were highly significant (p < 0.0001). Improvement in functionality was noted at 1 month and at end of treatment with significant reductions in total WOMAC score, individual pain, stiffness and physical function sub-scores (p < 0.0001). AEs causing discontinuation (n = 32) included nausea, dizziness and vomiting. Most treatment-related AEs were mild to moderate in intensity. TDF improved pain control, functionality and health-related quality of life in these patients. The findings support current recommendations for use of opioids such as TDF as a treatment option for a sub-population of patients with OA pain.





Similar content being viewed by others
References
Holbrook AM (2000) Ontario treatment guidelines for osteoarthritis, rheumatoid arthritis, and acute musculoskeletal injury. Queen’s Printer of Ontario, Toronto, Ontario ISBN:ISBN 1-894706-00-5
Peat G, Thomas E, Handy J et al (2004) The Knee Clinical Assessment Study—CAS(K). A prospective study of knee pain and knee osteoarthritis in the general population. BMC Musculoskelet Disord 5(1):4
Simon LS, Lipman AG, Jacox AK et al (2002) Guidelines for the management of Pain in Osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis, 2nd edn. APS, Glenview, IL
Jovey RD, Ennis J, Gardner-Nix J et al (2003) Use of opioid analgesics for the treatment of chronic noncancer pain – A consensus statement and guidelines from the Canadian Pain Society, 2002. Pain Res Manag 8(Suppl A):3A–14A
Ashburn MA, Lipman AG (2003) Principles of analgesic use in the treatment of acute pain and cancer pain, 5th edn. APS, Glenview, IL
Wolfe MM, Lichetenstein DR, Singh G (1999) Gastrointestinal toxicity of nonsteroidal anti-inflammatory drugs. N Engl J Med 340(24):1888–1899
Mamdani M, Juurlink DN, Lee DS et al (2004) Cyclo-oxygenase-2 inhibitors versus non-selective non-steroidal anti-inflammatory drugs and congestive heart failure outcomes in elderly patients: a population-based cohort study. Lancet 363(9423):1751–1756
Nussmeier NA, Whelton AA, Brown MT et al (2005) Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N Engl J Med 352(11):1081–1091
Milligan K, Lanteri-Minet M, Borchert K et al (2001) Evaluation of long-term efficacy and safety of transdermal fentanyl in the treatment of chronic noncancer pain. J Pain 2(4):197–204
Allan L, Hays H, Jensen NH et al (2001) Randomized cross over trial of transdermal fentanyl and sustained release oral morphine for treating chronic non-cancer pain. BMJ 322(7295):1154–1158
Caldwell JR, Hale ME, Boyd RE et al (1999) Treatment of osteoarthritis pain with controlled release oxycodone plus acetaminophen added to nonsteroidal antiinflammatory drugs: a double-blind, randomized placebo controlled trial. J Rheumatol 26:862–869
Roth SH, Fleischmann RM, Burch FX et al (2000) Around-the-clock, controlled-release oxycodone therapy for osteoarthritis-related pain: placebo-controlled trial and long-term evaluation. Arch Intern Med 160:853–860
Peloso PM, Bellamy N, Bensen W et al (2000) Double-blind randomized placebo-controlled trial of controlled-release codeine in the treatment of ostearthritis of the hip or knee. J Rheumatol 27:764–771
Theodoridis T, Waap I, Schwalen S et al (2003) Fentanyl-TTS in the treatment of pain caused by arthrosis [Article in German]. Z Orthop Ihre Grenzgeb 141(2):217–222
Simpson RK Jr, Edmondson EA, Constant CF et al (1997) Transdermal fentanyl as treatment for chronic low back pain. J Pain Symptom Manage 14(4):218–224
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29(8):1039–1049
Altman R, Alarcon G, Appelrouth D et al (1991) The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum 34(5):505–514
Bellamy N, Buchanan WW, Goldsmith CH et al (1996) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Ware JE, Sherbourne CD (1992) The MOS 36-Item Short-Form Health Status Survey (SF-36): Conceptual framework and item selection. Med Care 30(6):473–483
Ware JE, Snow KK, Kosinski M et al (1993) SF-36 Health Survey manual and interpretation guide. New England Medical Center, The Health Institute, Boston
Tuback F, Ravaud P et al (2005) Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis 64(1):29–33
Hägg O, Fritzell P et al (2003) The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J 12(1):12–20
Ostelo RW, de Vet HC (2005) Clinically important outcomes in low back pain: Best Pract Res Clin. Rheumatol 19(4):593–607
Mesrian A et al (2007) Reduction in pain intensity after treatment for chronic back pain. When is it clinically meaningful? Schmerz 21(3):212, 214–217
Angst F et al (2002) Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol 29(1):131–8
Weigl M et al (2006) Predictors for response to rehabilitation in patients with hip or knee osteoarthritis: a comparison of logistic regression models with three different definitions of responder.. Osteoarthritis Cartilage 14(7):641–651
Ferguson RJ et al (2002) Use of the Reliable Change Index to evaluate clinical significance in SF-36 outcomes.. Qual Life Res J 11(6):509–516
Wyrwich KW (1998) Linking clinical relevance and statistical significance in evaluating the responsiveness of quality of life instruments. Abstr Book Assoc Health Serv Res Meet 15:330
Schűnemann HJ, Guyatt GH (2005) Commentary—Goodbye M©ID! Hello MID, where do you come from? Health Serv Res 40(2):593–597
Langford R, McKenna F, Ratcliffe S et al (2006) Transdermal Fentanyl for Improvement of Pain and Functioning in Osteoarthritis. Arthritis Rheum 54(6):1829–1837
Le Loet X, Pavelka K, Richarz U (2005) Transdermal fentanyl for the treatment of pain caused by osteoarthritis of the knee or hip: an open, multicentre study. BMC Musculoskelet Disord 6:31
Clark AJ et al (2004) Efficacy and safety of Transdermal Fentanyl and sustained-release oral morphine in patients with cancer and chronic non-cancer pain. Curr Med Res Opin 20(9):1419–1428
Acknowledgements
The FEN-OAR-401 Study Group: Dr. Christopher Atkins (Victoria), Dr. Andre Beaulieu (Sainte-Foy), Dr. William Bensen (Hamilton), Dr. Denis Choquette (Montreal), Dr. Allan Kelly (Edmonton), Dr. Majed Khraishi (St. John’s), Dr. Timothy McCarthy (Winnipeg), Dr. Brian Ramjattan (St. John’s), Dr. Jude Rodrigues (Windsor), Dr. Michel Zummer (Montreal).
Additional support from Janssen-Ortho Inc. staff: J Dinniwell (study concept and design), A Gillespie-Wight, R Chawla, J Suzuki (data monitoring),: J Sigindere, D Estaris, F Dalzilio, R Lachhman (data support), B Teixeira (editorial support).
Author information
Authors and Affiliations
Corresponding author
Additional information
Contributors: Denis Choquette contributed to the concept and design of the study, participated in conducting the research, data analysis and co-wrote the manuscript. Fernando Camacho contributed statistical expertise to all phases of study design and data analysis and contributed to the content of the manuscript. Timothy G. McCarthy, Jude F.N. Rodrigues and Allan J. Kelly participated in conducting the research, contributed to data interpretation and to the content of the manuscript. Farah A. Husein-Bhabha coordinated the study, contributed to data analysis and manuscript preparation. G. L. A. Horbay contributed to the study design, data analysis and manuscript preparation. All authors read and approved the final manuscript.
Rights and permissions
About this article
Cite this article
Choquette, D., McCarthy, T.G., Rodrigues, J.F.N. et al. Transdermal fentanyl improves pain control and functionality in patients with osteoarthritis: an open-label Canadian trial. Clin Rheumatol 27, 587–595 (2008). https://doi.org/10.1007/s10067-007-0751-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0751-6