Abstract
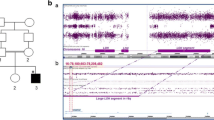
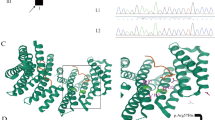
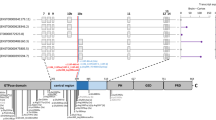
The human WWOX (WW domain-containing oxidoreductase) gene, originally known as a tumor suppressor gene, has been shown to be important for brain function and development. In recent years, mutations in WWOX have been associated with a wide phenotypic spectrum of autosomal recessively inherited neurodevelopmental disorders. Whole exome sequencing was completed followed by Sanger sequencing to verify segregation of the identified variants. Functional WWOX analysis was performed in fibroblasts of one patient. Transcription and translation were assessed by quantitative real-time PCR and Western blotting. We report two related patients who presented with early epilepsy refractory to treatment, progressive microcephaly, profound developmental delay, and brain MRI abnormalities. Additionally, one of the patients showed bilateral optic atrophy. Whole exome sequencing revealed homozygosity for a novel missense variant affecting the evolutionary conserved amino acid Gln230 in the catalytic short-chain dehydrogenase/reductase (SDR) domain of WWOX in both girls. Functional studies showed normal levels of WWOX transcripts but absence of WWOX protein. To our knowledge, our patients are the first individuals presenting the more severe end of the phenotypic spectrum of WWOX deficiency, although they were only affected by a single missense variant of WWOX. This could be explained by the functional data indicating an impaired translation or premature degradation of the WWOX protein.



Similar content being viewed by others
References
Aqeilan RI, Croce CM (2007) WWOX in biological control and tumorigenesis. J Cell Physiol 212(2):307–310
Chang HT, Liu CC, Chen ST, Yap YV, Chang NS, Sze CI (2014) WW domain-containing oxidoreductase in neuronal injury and neurological diseases. Oncotarget 5(23):11792
Chen ST, Chuang JI, Wang JP, Tsai MS, Li H, Chang NS (2014) Expression of WW domain-containing oxidoreductase WOX1 in the developing murine nervous system. Neuroscience 124(4):831–839
Tabarki B, Al Mutairi F, Al Hashem A (2014) The fragile site WWOX gene and the developing brain. Exp Biol Med 240(3):400–402
Sze CI, Kuo YM, Hsu LJ, Fu TF, Chiang MF, Chang JY, Chang NS (2015) A cascade of protein aggregation bombards mitochondria for neurodegeneration and apoptosis under WWOX deficiency. Cell Death Dis 6(9):e1881
Mallaret M, Synofzik M, Lee J, Sagum CA, Mahajnah M, Sharkia R (2014) The tumour suppressor gene WWOX is mutated in autosomal recessive cerebellar ataxia with epilepsy and mental retardation. Brain 137(2):411–419
Abdel-Salam G, Thoenes M, Afifi HH, Körber F, Swan D, Bolz HJ (2014) The supposed tumor suppressor gene WWOX is mutated in an early lethal microcephaly syndrome with epilepsy, growth retardation and retinal degeneration. Orphanet J Rare Dis 9(1):1
Ben-Salem S, Al-Shamsi AM, John A, Ali BR, Al-Gazali L (2015) A novel whole exon deletion in WWOX gene causes early epilepsy, intellectual disability and optic atrophy. J Mol Neurosci 56(1):17–23
Tabarki B, Al Hashem A, Al Shahwan S, Alkuraya FS, Gedela S, Zuccoli G (2015) Severe CNS involvement in WWOX mutations: description of five new cases. Am J Med Genet A 167(12):3209–3213
Mignot C, Lambert L, Pasquier L, Bienvenu T, Delahaye-Duriez A, Keren B, Lefranc J, Saunier A, Allou L, Roth V, Valduga M, Moustaïne A, Auvin S, Barrey C, Chantot-Bastaraud S, Lebrun N, Moutard ML, Nougues MC, Vermersch AI, Héron B, Pipiras E, Héron D, Olivier-Faivre L, Guéant JL, Jonveaux P, Philippe C (2015) WWOX-related encephalopathies: delineation of the phenotypical spectrum and emerging genotype-phenotype correlation. J Med Genet 52(1):61–70
Kortüm F, Caputo V, Bauer CK, Stella L, Ciolfi A, Alawi M, Bocchinfuso G, Flex E, Paolacci S, Dentici ML, Grammatico P, Korenke GC, Leuzzi V, Mowat D, Nair LD, Nguyen TT, Thierry P, White SM, Dallapiccola B, Pizzuti A, Campeau PM, Tartaglia M, Kutsche K (2015) Mutations in KCNH1 and ATP6V1B2 cause Zimmermann-Laband syndrome. Nat Genet 47(6):661–667
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30(15):2114–2120
Ioannidis NM, Rothstein JH, Pejaver V, Middha S, McDonnell SK, Baheti S, Musolf A, Li Q, Holzinger E, Karyadi D, Cannon-Albright LA, Teerlink CC, Stanford JL, Isaacs WB, Xu J, Cooney KA, Lange EM, Schleutker J, Carpten JD, Powell IJ, Cussenot O, Cancel-Tassin G, Giles GG, MacInnis RJ, Maier C, Hsieh CL, Wiklund F, Catalona WJ, Foulkes WD, Mandal D, Eeles RA, Kote-Jarai Z, Bustamante CD, Schaid DJ, Hastie T, Ostrander EA, Bailey-Wilson JE, Radivojac P, Thibodeau SN, Whittemore AS, Sieh W (2016) REVEL: an ensemble method for predicting the pathogenicity of rare missense variants. Am J Hum Genet 99(4):877–885
Jagadeesh KA, Wenger AM, Berger MJ, Guturu H, Stenson PD, Cooper DN, Bernstein JA, Bejerano G (2016) M-CAP eliminates a majority of variants of uncertain significance in clinical exomes at high sensitivity. Nat Genet 48(12):1581–1586
Kircher M, Witten DM, Jain P, O'roak BJ, Cooper GM, Shendure J (2014) A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet 46(3):310–315
Schirmer MA, Lüske CM, Roppel S, Schaudinn A, Zimmer C, Pflüger R, Haubrock M, Rapp J, Güngör C, Bockhorn M, Hackert T, Hank T, Strobel O, Werner J, Izbicki JR, Johnson SA, Gaedcke J, Brockmöller J, Ghadimi BM (2016) Relevance of Sp binding site polymorphism in WWOX for treatment outcome in pancreatic cancer. JNCI 108(5):djv387
Wang X, Spandidos A, Wang H, Seed B (2011) PrimerBank: a PCR primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res 40(D1):D1144–D1149
MacArthur MW, Thornton JM (1991) Influence of proline residues on protein conformation. J Mol Biol 218(2):397–412
Hetz C, Mollereau B (2014) Disturbance of endoplasmic reticulum proteostasis in neurodegenerative diseases. Nat Rev Neurosci 15(4):233–249
Stefl S, Nishib H, Petukha M, Panchenkob AR, Alexova (2013) Molecular mechanisms of disease-causing missense mutations. J Mol Biol 425(21):3919–3936
Acknowledgements
We are thankful to the affected individuals and their families for participation.
Contributorship Statement
JJ collected the clinical data and drafted the article. FK and GR performed genetic analyses. KB and MAS provided functional analysis. All authors interpreted data. FK, MAS, JD, RS revised the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Johannsen, J., Kortüm, F., Rosenberger, G. et al. A novel missense variant in the SDR domain of the WWOX gene leads to complete loss of WWOX protein with early-onset epileptic encephalopathy and severe developmental delay. Neurogenetics 19, 151–156 (2018). https://doi.org/10.1007/s10048-018-0549-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-018-0549-5