Abstract
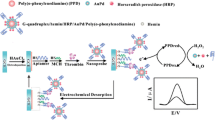
A highly sensitive label-free electrochemical aptasensor has been constructed for the electrochemical detection of thrombin (TB), where two layers of cobalt hexacyanoferrate (CoHCF) redox probes sandwiched with carbon nanotubes–Nafion were directly immobilized on the electrode surface by electrodeposition. Through the strong interaction between CN− (CoHCF) and gold nanoparticles (GNPs), GNPs were assembled on the CoHCF-modified electrode for the immobilization of thiolated thrombin aptamers (TBA). In the presence of target TB, TBA on the electrode surface could catch TB to form TBA–TB complex, which made a barrier for the electron transfer, resulting in a greater decrease in CoHCF redox probe signals. Thus, the proposed aptasensor showed a high sensitivity and a much wider linearity to TB in the range of 1.0 pg/mL ∼ 1.0 μg/mL with a detection limit of 0.28 pg/mL.









Similar content being viewed by others
References
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Famulok M, Hartig JS, Mayer G (2007) Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem Rev 107:3715–3743
Peng YG, Zhang DD, Li Y, Qi HL, Gao Q, Zhang CX (2009) Label-free and sensitive faradic impedance aptasensor for the determination of lysozyme basedon target-induced aptamer displacement. Biosens Bioelectron 25:94–99
Hansen JA, Wang J, Kawde AN, Xiang Y, Gothelf KV, Collins G (2006) Quantum-dot/aptamer-based ultrasensitive multi-analyte electrochemical biosensor. J Am Chem Soc 128:2228–2229
Chai Y, Tian DY, Cui H (2012) Electrochemiluminescence biosensor for the assay of small molecule and protein based on bifunctionalaptamer and chemiluminescent functionalized gold nanoparticles. Anal Chim Acta 715:86–92
Luo F, Zheng LY, Chen SS, Cai QH, Lin ZY, Qiu B, Chen GN (2012) An aptamer-based fluorescence biosensor for multiplex detection using unmodified gold nanoparticles. Chem Commun 48:6387–6389
Jiang L, Yuan R, Chai Y, Yuan Y, Bai L, Wang Y (2012) Aptamer-based highly sensitive electrochemical detection of thrombin via the amplification of graphene. Analyst 137:2415–2420
Mir M, Vreeke M, Katakis I (2006) Different strategies to develop an electrochemical thrombin aptasensor. Electrochem Commun 8:505–511
Wang L, Zhu C, Han L, Jin L, Zhou M, Dong S (2011) Label-free, regenerative and sensitive surface plasmon resonance and electrochemical aptasensors based on graphene. Chem Commun 47:7794–7796
Bai L, Yuan R, Chai Y, Yuan Y, Mao L, Zhou Y (2011) Highly sensitive electrochemical label-free aptasensor based on dual electrocatalytic amplification of Pt-AuNPs and HRP. Analyst 136:1840–1845
Tacconi NR, Rajeshwar K (2003) Metalhexacyanoferrate: electrosynthesis, in situ characterization and applications. Chem Mater 15:3046–3062
Li J, Qiu JD, Xu JJ, Chen HY, Xi XH (2007) The Synergistic effect of prussia-blue-grafted carbon nanotube/poly(4-vinylpyridine) composites for amperometric sensing. Adv Funct Mater 17:1574–1580
Chen SM (2002) Preparation, characterization, and electrocatalytic oxidation properties of iron, cobalt, nickel, and indium hexacyanoferrate. J Electroanal Chem 521:29–52
Chen SM, Chan CM (2003) Preparation, characterization, and electrocatalytic properties of copper hexacyanoferrate film and bilayer film modified electrodes. J Electroanal Chem 543:161–173
Florescu M, Barsan M, Pauliukaite R, Bretta CMA (2007) Development and application of oxysilane sol–gel electrochemical glucose biosensors based on cobalthexacyanoferrate modified carbon film electrodes. Electroanalysis 19:220–226
Berrettoni M, Gioregetti M, Cox JA, Ranganathan D, Conti P, Zamponi S (2012) Electrochemical synthesis of nano-cobalt hexacyanoferrate at a sol–gel-coated electrode templated with β-cyclodextrin. J Solid State Electrochem 16:2861–2866
Senthil Kumar S, Sriman Narayanan S (2006) Amperometric sensor for the determination of ascorbic acid based on cobalt hexacyanoferrate modified electrode fabricated through a new route. Chem Pharm Bull 54:963–967
Yan X, Pan D, Wang H, Bo X, Guo L (2011) Electrochemical determination of L-dopa at cobalt hexacyanoferrate/large-mesopore carbon composite modified electrode. J Electroanal Chem 663:36–42
Li X, Chen Z, Zhong Y, Yang F, Pan J, Liang Y (2012) Cobalt hexacyanoferrate modified multi-walled carbon nanotubes/graphite composite electrode as electrochemical sensor on microfluidic chip. Anal Chim Acta 710:118–124
Yang M, Jiang J, Yang Y, Chen X, Shen G, Yu R (2006) Carbon nanotube/cobalt hexacyanoferrate nanoparticle-biopolymer system for the fabrication of biosensors. Biosens Bioelectron 21:1791–1797
Pauliukaite R, Florescu M, Brett CMA (2005) Characterization of cobalt-and copper hexacyanoferrate-modified carbon film electrodes for redox-mediated biosensors. J Solid State Electrochem 9:354–362
Pingarron JM, Yanez-Sedeno P, Gonzalez-Cortes A (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848–5866
Wang J, Li SP, Zhang YZ (2010) A sensitive DNA biosensor fabricated from gold nanoparticles, carbon nanotubes, and zinc oxide nanowires on a glassy carbon electrode. Electrochim Acta 55:4436–4440
Li L, Zhao H, Chen Z, Mu X, Guo L (2010) Aptamer-based electrochemical approach to the detection of thrombin by modification of gold nanoparticles. Anal Bioanal Chem 398:563–570
Zheng J, Lin L, Cheng GF, Wang AB, Tan XL, He PG, Fang YZ (2007) Study on an electrochemical biosensor for thrombin recognition based on aptamers and nano particles. Sci China Ser B 50:351–357
Prieto-Simon B, Campas M, Marty JL (2010) Electrochemical aptamer-based sensors. Bioanal Rev 1:141–157
Qi YY, Li BX (2011) A Sensitive, Label-free, aptamer-based biosensor using a gold nanoparticleinitiated chemiluminescence system. Chem Eur J 17:1642–1648
Zargoosh K, Chaichi MJ, Shamsipur M, Hossienkhani S, Asghari S, Qandalee M (2012) Highly sensitive glucose biosensor based on the effective immobilization of glucose oxidase/carbon-nanotube and gold nanoparticle in nafion film and peroxyoxalate chemiluminescence reaction of a new fluorophore. Talanta 93:37–43
Mir M, Jenkins ATA, Katakis I (2008) Ultrasensitive detection based on an aptamer beacon electron transfer chain. Electrochem Commun 10:1533–1536
Frens G (1973) Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nat Phys Sci 241:20–22
Turkevich J, Stevenson PC, Hillier J (1951) A study of the nucleation and growth processes in the synthesis of colloidal gold. Disc Faraday Soc 11:55–75
Liu M, Li P, Cheng Y, Xian Y, Zhang C, Jin L (2004) Determination of thiol compounds in rat striatum microdialysate by HPLC with a nanosized CoHCF-modified electrode. Anal Bioanal Chem 380:742–750
Pournaghi-Azar MH, Sabzi R (2002) Preparation of a cobalt hexacyanoferrate film-modified aluminum electrode by chemical and electrochemical methods: enhanced stability of the electrode in the presence of phosphate and ruthenium(III). J Solid State Electrochem 6:553–559
Ravishankaran D, Sriman Narayanan S (2002) Amperometric sensor for thiosulphate based on cobalt hexacyanoferrate modified electrode. Sensors Actuators B 86:180–184
Deng C, Chen J, Nie Z, Wang M, Chu X, Chen X, Xiao X, Lei C, Yao S (2009) Impedimetric aptasensor with femtomolar sensitivity based on the enlargement of surface-charged gold nanoparticles. Anal Chem 81:739–745
Xu D, Yu X, Liu Z, He W, Ma Z (2005) Label-free electrochemical detection for aptamer-based array electrodes. Anal Chem 77:5107–5113
Sun Y, Bai Y, Yang W, Sun C (2007) Controlled multilayer films of sulfonate-capped gold nanoparticles/thionine used for construction of a reagentless bienzymatic glucose biosensor. Electrochim Acta 52:7352–7361
Li LD, Zhao HT, Chen ZB, Mu XJ, Guo L (2011) Aptamer biosensor for label-free impedance spectroscopy detection of thrombin based on gold nanoparticles. Sensors Actuators B 157:189–194
Yang H, Ji J, Liu Y, Kong J, Liu B (2009) An aptamer-based biosensor for sensitive thrombin detection. Electrochem Commun 11:38–40
Xie S, Yuan R, Chai Y, Bai L, Yuan Y, Wang Y (2012) Label-free electrochemical aptasensor for sensitive thrombin detection using layer-by-layer self-assembled multilayers with toluidine blue–graphene composites and gold nanoparticles. Talanta 98:7–13
Zhao J, Zhang Y, Li H, Wen Y, Fan X, Lin F, Tan L, Yao S (2011) Ultrasensitive electrochemical aptasensor for thrombin based on the amplification of aptamer–AuNPs–HRP conjugates. Biosens Bioelectron 26:2297–2303
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21065004), Natural Science Foundation of Jiangxi Province of China (2009GQH0022), and Scientific Research Fund of Jiangxi Provincial Education Department (GJJ12304).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 821 kb)
Rights and permissions
About this article
Cite this article
Yang, S., Li, H., Zha, W. et al. Highly sensitive lable-free electrochemical aptasensor for thrombin detection with cobalt hexacyanoferrate as the electrochemical probe. J Solid State Electrochem 17, 2603–2610 (2013). https://doi.org/10.1007/s10008-013-2133-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2133-0