Abstract
Context
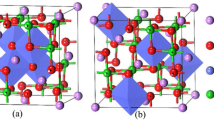
Ni-rich layered oxides have been widely studied as cathodes because of their high energy density. However, the gradual structural transformation during the cycle will lead to the capacity degradation and potential decay of the cathode materials. In this paper, first-principle calculations were used to investigate the formation energy, and geometric and electronic structure of Mg-doped LiNiO2 cathode for Li-ion batteries. The results show that Mg doping has little effect on the geometric structure of LiNiO2 but has great effect on its electronic structure. Our data give an insight into the microscopic mechanism of Mg-doped LiNiO2 and provide a theoretical reference for experimental research, which is helpful to the design of safer and higher energy density Ni-rich cathodes.
Method
In this work, all calculations were performed by the VASP package; the PBE functional in the generalized gradient approximation (GGA) was employed to describe the exchange–correlation interactions. An energy cutoff of 520 eV and a 5 × 5 × 3 Monkhorst–Pack mesh of k-point sampling in the Brillouin zone were chosen for all calculations. All atoms were relaxed until the convergences of 10−5 eV/f.u in energy and 0.01 eV/Å in force were reached.






Similar content being viewed by others
Data availability
The data generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Goodenough JB, Park KS (2013) J Am Chem Soc 135:1167
Zhang L, Wang S, Wang Q, Shao H, Jin Z (2023) Adv Mater 35:2303355
Sun L, Xie J, Jin Z (2019) Energy Technol 7:1900962
Lu L, Han X, Li J, Hua J, Ouyang M (2013) J Power Source 226:272
Goodenough JB, Kim Y (2010) Chem Mater 22:587
Etacheri V, Marom R, Elazari R, Salitra G, Aurbach D (2011) Energy Environ Sci 4:3243
Sun L, Liu Y, Shao R, Wu J, Jiang R, Jin Z (2022) Energy Storage Mater 46:482
Wang Y, Liang J, Song X, Jin Z (2023) Energy Storage Mater 54:732
Kalyani P, Kalaiselvi N (2005) Sci Technol Adv Mater 6:689
Bianchini M, Roca-Ayats M, Hartmann P, Brezesinski T, Janek J (2019) Angew Chem Int Ed 58:10434
Zhao E, Fang L, Chen M, Chen D, Huang Q, Hu Z, Yan QB, Wu M, Xiao X (2017) J Mater Chem A 5:1679
Choi D, Kang J, Han B (2019) Electrochim Acta 294:166
Kang J, Han B (2015) Acs Appl Mater Interfaces 7:11599
Ohzuku T, Ueda A, Nagayama M (1993) J Electrochem Soc 24:1862
Urban A, Abdellahi A, Dacek S, Artrith N, Ceder G (2017) Phys Rev Lett 119:176402
Duan J, Hu G, Cao Y, Tan C, Wu C, Du K, Peng Z (2016) J Power Source 326:322
Dianat A, Seriani N, Bobeth M, Cuniberti G (2013) J Mater Chem A 1:9273
Hong-Bin L, Chun L, Yue C, Ke-Hua Z, Jian-Min Z, Gui-Gui X, Zhi-Gao H (2021) Acta Phy Sin-CH ED 70:138201
Shim JH, Kim YM, Park M, Kim J, Lee S (2017) Acs Appl Mater Interfaces 9:18720
Xiong X, Wang Z, Yan G, Guo H, Li X (2014) J Power Sources 245:183
Chen Z, Qin Y, Amine K, Sun YK (2010) J Mater Chem 20:7606
Song MY, Lee DS, Park HR (2012) Mater Res Bull 47:1021
Huang GX, Wang RH, Lv XY, Su J, Long YF, Qin ZZ, Wen YX (2022) J Electrochem Soc 169:040533
Kong F, Liang C, Longo RC, Yeon DH, Zheng Y, Park JH, Doo SG, Cho K (2016) Chem Mater 28:6942
Gomez-Martin A, Reissig F, Frankenstein L, Heidbüchel M, Winter M, Placke T, Schmuch R (2022) Adv Energy Mater 12:2103045
Kresse G, Hafner J (1993) Phys Rev B 47:558
Kresse G, Furthmüller J (1996) Comp Mater Sci 6:15
Kresse G, Furthmüller J (1996) Phys Rev B 54:11169
Jain A, Hautier G, Ong SP, Moore CJ, Fischer CC, Persson KA, Ceder G (2011) Phys Rev B 84:045115
Xiao P, Deng ZQ, Manthiram A, Henkelman G (2012) J Phys Chem C 116:23201
Hu W, Kou H, Chen Y, Wang Y, Zhu H, Li G, Li H (2022) Colloid Surface A 648:129185
Vallverdu G, Minvielle M, Andreu N, Gonbeau D, Baraille I (2016) Surf Sci 649:46
Dyer LD, Borie BS, Smith GP (1954) J Am Chem Soc 76:1499
Seong WM, Manthiram A (2020) Acs Appl Mater Interfaces 12:43653
Kanno R, Kubo H, Kawamoto Y, Kamiyama T, Izumi F, Takeda Y, Takano M (1994) J Solid State Chem 110:216
Fang L, Wang M, Zhou Q, Xu H, Hu W, Li H (2020) Colloid Surface A 600:124940
Funding
This work was supported by Science and Technology Project of Jiangxi Provincial Department of Education (Grant No. GJJ211215) and Jiangxi University of Chinese Medicine Science and Technology Innovation Team Development Program (No: CXTD22015).
Author information
Authors and Affiliations
Contributions
HL: conceptualization, writing—original draft preparation, writing—review; YZ: methodology, investigation; QY: formal analysis; WH: resources, supervision; QZ: formal analysis, editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, H., Zhu, Y., Ye, Q. et al. First-principle study on the geometric and electronic structure of Mg-doped LiNiO2 for Li-ion batteries. J Mol Model 29, 389 (2023). https://doi.org/10.1007/s00894-023-05797-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05797-w