Abstract
Context
Ampicillin (AMP) is a penicillin-class beta-lactam antibiotic widely used to treat infections caused by bacteria. Therefore, due to its widespread use, this antibiotic is found in wastewater, and it contains long-term risks such as toxicity to all living organisms.
Method
In this study, the degradation reaction of ampicillin with hydroxyl radical was investigated by the density functional theory (DFT) method. All the calculations were performed with B3LYP functional at 6-31G(d,p) basis set.
Results
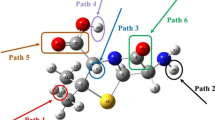
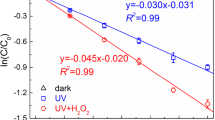
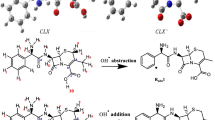
The thermodynamic energy values and reaction rates of all possible reaction paths were calculated. The addition of the hydroxyl radical to the carbonyl group of the beta-lactam ring is thermodynamically the most probable reaction path. The calculated overall reaction rate constant is 1.36 × 1011 M−1 s−1. To determine the effect of temperature on the reaction rate, rate constants were calculated for all reaction paths at five different temperatures. The subsequent reaction kinetics of the most preferred primary route was also examined, and the toxicity values of the intermediates were estimated. The acute toxicity of AMP and its degradation product were calculated using the Ecological Structure Activity Relationships (ECOSAR) software. The degradation product was found to be more toxic than AMP.








Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article (and its supplementary information files).
References
Shukla A, Khan E, Tandon P, Sinha K (2017) Study of vibrational spectra and hydrogen bonding network in dimeric and tetrameric model of ampicillin using DFT and AIM approach. J Mol Struct 1131:225–235. https://doi.org/10.1016/j.molstruc.2016.11.057
He X, Mezyk SP, Michael I et al (2014) Degradation kinetics and mechanism of β-lactam antibiotics by the activation of H2O2 and Na2S2O8 under UV-254nm irradiation. J Hazard Mater 279:375–383. https://doi.org/10.1016/j.jhazmat.2014.07.008
Serna-Galvis EA, Cáceres-Peña AC, Torres-Palma RA (2020) Elimination of representative fluoroquinolones, penicillins, and cephalosporins by solar photo-Fenton: degradation routes, primary transformations, degradation improvement by citric acid addition, and antimicrobial activity evolution. Environ Sci Pollut Res 27:41381–41393. https://doi.org/10.1007/s11356-020-10069-8
Xu L, Li W, Désesquelles P et al (2019) A statistical model and DFT study of the fragmentation mechanisms of metronidazole by advanced oxidation processes. J Phys Chem A 123:933–942. https://doi.org/10.1021/acs.jpca.8b10554
Ioannou-Ttofa L, Raj S, Prakash H, Fatta-Kassinos D (2019) Solar photo-Fenton oxidation for the removal of ampicillin, total cultivable and resistant E. coli and ecotoxicity from secondary-treated wastewater effluents. Chem Eng J 355:91–102. https://doi.org/10.1016/j.cej.2018.08.057
Darwish M, Mohammadi A, Assi N (2016) Integration of nickel doping with loading on graphene for enhanced adsorptive and catalytic properties of CdS nanoparticles towards visible light degradation of some antibiotics. J Hazard Mater 320:304–314. https://doi.org/10.1016/j.jhazmat.2016.08.043
Rozas O, Contreras D, Mondaca MA et al (2010) Experimental design of Fenton and photo-Fenton reactions for the treatment of ampicillin solutions. J Hazard Mater 177:1025–1030. https://doi.org/10.1016/j.jhazmat.2010.01.023
Sharma VK, Liu F, Tolan S et al (2013) Oxidation of β-lactam antibiotics by ferrate(VI). Chem Eng J 221:446–451. https://doi.org/10.1016/j.cej.2013.02.024
Shukla A, Khan E, Srivastava A et al (2016) A computational study on molecular structure, multiple interactions, chemical reactivity and molecular docking studies on 6[D (−) α-amino-phenyl-acetamido] penicillanic acid (ampicillin). Mol Simul 42:863–873. https://doi.org/10.1080/08927022.2015.1089996
Vidal J, Huiliñir C, Santander R et al (2019) Degradation of ampicillin antibiotic by electrochemical processes: evaluation of antimicrobial activity of treated water. Environ Sci Pollut Res 26:4404–4414. https://doi.org/10.1007/s11356-018-2234-5
Serna-Galvis EA, Montoya-Rodríguez D, Isaza-Pineda L et al (2019) Sonochemical degradation of antibiotics from representative classes—considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason Sonochem 50:157–165. https://doi.org/10.1016/j.ultsonch.2018.09.012
Belhacova L, Bibova H, Marikova T et al (2021) Removal of ampicillin by heterogeneous photocatalysis: combined experimental and dft study. Nanomaterials 11. https://doi.org/10.3390/nano11081992
Timm A, Borowska E, Majewsky M et al (2019) Photolysis of four β-lactam antibiotics under simulated environmental conditions: degradation, transformation products and antibacterial activity. Sci Total Environ 651:1605–1612. https://doi.org/10.1016/j.scitotenv.2018.09.248
Yabalak E (2018) An approach to apply eco-friendly subcritical water oxidation method in the mineralization of the antibiotic ampicillin. J Environ Chem Eng 6:7132–7137. https://doi.org/10.1016/j.jece.2018.10.010
Li T, Xu X, Fu S et al (2014) Structural elucidation of stress degradation products of ampicillin sodium by liquid chromatography/hybrid triple quadrupole linear ion trap mass spectrometry and liquid chromatography/hybrid quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 28:1929–1936. https://doi.org/10.1002/rcm.6970
Ribeiro AR, Sures B, Schmidt TC (2018) Cephalosporin antibiotics in the aquatic environment: a critical review of occurrence, fate, ecotoxicity and removal technologies. Environ Pollut 241:1153–1166
Elmolla ES, Chaudhuri M (2010) Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination 252:46–52. https://doi.org/10.1016/j.desal.2009.11.003
Szabó L, Tóth T, Engelhardt T et al (2016) Change in hydrophilicity of penicillins during advanced oxidation by radiolytically generated OH compromises the elimination of selective pressure on bacterial strains. Sci Total Environ 551–552:393–403. https://doi.org/10.1016/j.scitotenv.2016.02.002
Yao J, Tang Y, Zhang Y et al (2022) New theoretical investigation of mechanism, kinetics, and toxicity in the degradation of dimetridazole and ornidazole by hydroxyl radicals in aqueous phase. J Hazard Mater 422. https://doi.org/10.1016/j.jhazmat.2021.126930
Xu M, Yan S, Sun S et al (2022) N, N -diethyl-m-toluamide (DEET) degradation by •oH and SO4•--assisted AOPs in wastewater treatment: theoretical studies into mechanisms, kinetics and toxicity. J Environ Chem Eng 10. https://doi.org/10.1016/j.jece.2022.108435
Xu M, Yao J, Sun S et al (2021) Theoretical calculation on the reaction mechanisms, kinetics and toxicity of acetaminophen degradation initiated by hydroxyl and sulfate radicals in the aqueous phase. Toxics 9. https://doi.org/10.3390/toxics9100234
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta Jr. JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision C.01 Gaussian, Inc., Wallingford
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates Cartesians and with internal coordinates without mass-weighting. J Phys Chem 94:5523. https://doi.org/10.1021/j100377a021
Foresman J, Frish E (1996) Exploring chemistry. Gaussian Inc, Pittsburg
Dennington R, Keith TA, Millam JM (2016) GaussView, Version 5.0. Semichem Inc., Shawnee Mission, KS
Saїd AE hadj, Mekelleche SM (2021) Antioxidant activity of Trolox derivatives toward methylperoxyl radicals: thermodynamic and kinetic theoretical study. Theor Chem Acc 140. https://doi.org/10.1007/s00214-021-02815-z
Levine IN (2009) Physical Chemistry, 6th edn. Mc Graw Hill Higher Education, New York
Yang J, Lv G, Li T et al (2022) Theoretical insight into the degradation of diclofenac by hydroxyl and sulfate radicals in aqueous-phase: Mechanisms, kinetics and eco-toxicity. J Environ Chem Eng 10. https://doi.org/10.1016/j.jece.2022.108311
Sanches-Neto FO, Ramos B, Lastre-Acosta AM et al (2021) Aqueous picloram degradation by hydroxyl radicals: unveiling mechanism, kinetics, and ecotoxicity through experimental and theoretical approaches. Chemosphere 278. https://doi.org/10.1016/j.chemosphere.2021.130401
Milenković DA, Dimić DS, Avdović EH et al (2020) Advanced oxidation process of coumarins by hydroxyl radical: towards the new mechanism leading to less toxic products. Chem Eng J 395. https://doi.org/10.1016/j.cej.2020.124971
Tay KS, Madehi N (2015) Ozonation of ofloxacin in water: by-products, degradation pathway and ecotoxicity assessment. Sci Total Environ 520:23–31. https://doi.org/10.1016/j.scitotenv.2015.03.033
Bekbolet M, Çinar Z, Kiliç M et al (2009) Photocatalytic oxidation of dinitronaphthalenes: theory and experiment. Chemosphere 75:1008–1014. https://doi.org/10.1016/j.chemosphere.2009.01.051
Dail MK, Mezyk SP (2010) Hydroxyl-radical-induced degradative oxidation of β-lactam antibiotics in water: absolute rate constant measurements. J Phys Chem A 114:8391–8395. https://doi.org/10.1021/jp104509t
Antonin VS, Aquino JM, Silva BF et al (2019) Comparative study on the degradation of cephalexin by four electrochemical advanced oxidation processes: evolution of oxidation intermediates and antimicrobial activity. Chem Eng J 372:1104–1112. https://doi.org/10.1016/j.cej.2019.04.185
Song W, Chen W, Cooper WJ et al (2008) Free-radical destruction of β-lactam antibiotics in aqueous solution. J Phys Chem A 112:7411–7417. https://doi.org/10.1021/jp803229a
Vione D, de Laurentiis E, Berto S et al (2016) Modeling the photochemical transformation of nitrobenzene under conditions relevant to sunlit surface waters: reaction pathways and formation of intermediates. Chemosphere 145:277–283. https://doi.org/10.1016/j.chemosphere.2015.11.039
Kovacevic G, Sabljic A (2013) Theoretical study on the mechanism and kinetics of addition of hydroxyl radicals to fluorobenzene. J Comput Chem 34:646–655. https://doi.org/10.1002/jcc.23175
Kovacevic G, Sabljic A (2013) Mechanisms and reaction-path dynamics of hydroxyl radical reactions with aromatic hydrocarbons: the case of chlorobenzene. Chemosphere 92:851–856. https://doi.org/10.1016/j.chemosphere.2013.04.041
Li C, Zheng S, Chen J et al (2018) Kinetics and mechanism of [rad]OH-initiated atmospheric oxidation of organophosphorus plasticizers: a computational study on tri-p-cresyl phosphate. Chemosphere 201:557–563. https://doi.org/10.1016/j.chemosphere.2018.03.034
Hatipoglu A, Vione D, Yalçin Y et al (2010) Photo-oxidative degradation of toluene in aqueous media by hydroxyl radicals. J Photochem Photobiol A Chem 215:59–68. https://doi.org/10.1016/j.jphotochem.2010.07.021
Aydogdu S, Hatipoglu A, Eren B, Gurkan YY (2021) Photodegradation kinetics of organophosphorous with hydroxyl radicals: experimental and theoretical study. J Serb Chem Soc 86:955–969. https://doi.org/10.2298/JSC210409056A
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338. https://doi.org/10.1021/ja01607a027
Xia H, Zhang W, Yang Y et al (2021) Degradation mechanism of tris(2-chloroethyl) phosphate (TCEP) as an emerging contaminant in advanced oxidation processes: A DFT modelling approach. Chemosphere 273. https://doi.org/10.1016/j.chemosphere.2021.129674
Guo Y, Tsang DCW, Zhang X, Yang X (2018) Cu(II)-catalyzed degradation of ampicillin: effect of pH and dissolved oxygen. Environ Sci Pollut Res 25:4279–4288. https://doi.org/10.1007/s11356-017-0524-y
Sohrabi A, Haghighat G, Shaibani PM et al (2019) Degradation of pharmaceutical contaminants in water by an advanced plasma treatment. Desalination Water Treat 139:202–221. https://doi.org/10.5004/dwt.2019.23297
Mirzaei A, Haghighat F, Chen Z, Yerushalmi L (2019) Sonocatalytic removal of ampicillin by Zn(OH)F: effect of operating parameters, toxicological evaluation and by-products identification. J Hazard Mater 375:86–95. https://doi.org/10.1016/j.jhazmat.2019.04.069
Peterson JW, Petrasky LJ, Seymour MD et al (2012) Adsorption and breakdown of penicillin antibiotic in the presence of titanium oxide nanoparticles in water. Chemosphere 87:911–917. https://doi.org/10.1016/j.chemosphere.2012.01.044
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Gao Y, Ji Y, Li G, An T (2014) Mechanism, kinetics and toxicity assessment of OH-initiated transformation of triclosan in aquatic environments. Water Res 49:360–370. https://doi.org/10.1016/j.watres.2013.10.027
Gour NK, Borthakur K, Paul S, Chandra Deka R (2020) Tropospheric degradation of 2-fluoropropene (CH3CF[dbnd]CH2) initiated by hydroxyl radical: reaction mechanisms, kinetics and atmospheric implications from DFT study. Chemosphere 238. https://doi.org/10.1016/j.chemosphere.2019.124556
Arathala P, Musah RA (2020) Computational study investigating the atmospheric oxidation mechanism and kinetics of dipropyl thiosulfinate initiated by OH radicals and the fate of propanethiyl radical. J Phys Chem A 124:8292–8304. https://doi.org/10.1021/acs.jpca.0c05200
Gao Y, An T, Fang H et al (2014) Computational consideration on advanced oxidation degradation of phenolic preservative, methylparaben, in water: mechanisms, kinetics, and toxicity assessments. J Hazard Mater 278:417–425. https://doi.org/10.1016/j.jhazmat.2014.05.081
Burns JM, Cooper WJ, Ferry JL et al (2012) Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat Sci 74:683–734. https://doi.org/10.1007/s00027-012-0251-x
Klasmeier J, Matthies M, Macleod M et al (2006) Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ Sci Technol 40:53–60. https://doi.org/10.1021/es0512024
Funding
This study is supported by the Yildiz Technical University Research Coordination with project number FDK-2021–4138. We also received computational resources support from the Tubitak TRUBA.
Author information
Authors and Affiliations
Contributions
Arzu Hatipoglu: conceptualization, writing — reviewing and editing; Seyda Aydogdu: DFT calculations, writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aydogdu, S., Hatipoglu, A. Theoretical insights into the reaction mechanism and kinetics of ampicillin degradation with hydroxyl radical. J Mol Model 29, 63 (2023). https://doi.org/10.1007/s00894-023-05462-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-023-05462-2