Abstract
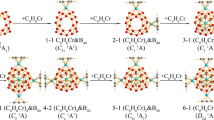
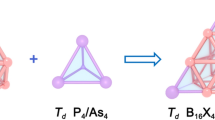
Cage-like and core-shell metallo-borospherenes exhibit interesting structures and bonding. Based on extensive global searches and first-principles theory calculations, we predict herein the perfect tetrahedral cage-like Td La4B24 (1) and core-shell Td La4B29 (2), Td La4B29+ (3), and Td La4B29− (4) which all possess the same geometrical symmetry as their carbon fullerene counterpart Td C28, with four equivalent interconnected B6 triangles on the cage surface and four nona-coordinate La centers in four conjoined η9-B9 rings. In these tetra-La-doped boron complexes, La4[B@B4@B24]0/+/− (2/3/4) in the structural motif of 1 + 4 + 28 contain a B-centered tetrahedral Td B@B4 core in a La-decorated tetrahedral La4B24 shell, with the negatively charged tetra-coordinate B− at the center being the boron analog of tetrahedral C in Td CH4 (B− ~ C). Detailed orbital and bonding analyses indicate that these Td lanthanide boride complexes are spherically aromatic in nature with a universal La--B9 (d-p) σ and (d-p) δ coordination bonding pattern. The IR, Raman, and UV-Vis or photoelectron spectra of these novel metallo-borospherenes are computationally simulated to facilitate their spectral characterizations.

Graphical abstract




Similar content being viewed by others
References
Cotton FA, Wilkinson G, Murrillo CA, Bochmann M, Advanced Inorganic Chemistry, Wiley, New York, 6th edn, 1999, p. 1355, ISBN 0-471-19957-5
Wang LS (2016) Photoelectron spectroscopy of size-selected boron clusters: from planar structures to borophenes and borospherenes. Int. Rev. Phys. Chem. 35:69–142. https://doi.org/10.1080/0144235X.2016.1147816
Jian T, Chen XN, Li SD, Boldyrev AI, Li J, Wang LS (2019) Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 48:3550–3591. https://doi.org/10.1039/c9cs00233b
Chen Q, Li WL, Zhao YF, Zhang SY, Hu HS, Bai H, Li HR, Tian WJ, Lu HG, Zhai HJ, Li SD, Li J, Wang LS (2015) Experimental and theoretical evidence of an axially chiral Borospherene. ACS Nano 9:754–760. https://doi.org/10.1021/nn506262c
Zhai HJ, Zhao YF, Li WL, Chen Q, Bai H, Hu HS, Piazza ZA, Tian WJ, Lu HG, Wu YB, Mu YW, Wei GF, Liu ZP, Li J, Li SD, Wang LS (2014) Observation of an all-boron fullerene. Nat. Chem. 6:727–731. https://doi.org/10.1038/nchem.1999
Bai H, Chen TT, Chen Q, Zhao XY, Zhang YY, Chen WJ, Li WL, Cheung LF, Bai B, Cavanagh J, Huang W, Li SD, Li J, Wang LS (2019) Planar B41− and B42− clusters with double-hexagonal vacancies. Nanoscale 11:23286–23295. https://doi.org/10.1039/C9NR09522E
Wang YJ, Zhao YF, Li WL, Jian T, Chen Q, You XR, Ou T, Zhao XY, Zhai HJ, Li SD, Li J, Wang LS (2016) Observation and characterization of the smallest borospherene, B28− and B28. J. Chem. Phys. 144:064307. https://doi.org/10.1063/1.4941380
Li HR, Jian T, Li WL, Miao CQ, Wang YJ, Chen Q, Luo XM, Wang K, Zhai HJ, Li SD, Wang LS (2016) Competition between quasi-planar and cage-like structures in the B29 cluster: photoelectron spectroscopy and ab initio calculations. Phys. Chem. Chem. Phys. 18:29147–29155. https://doi.org/10.1039/C6CP05420J
Bai H, Chen Q, Zhai HJ, Li SD (2015) Endohedral and Exohedral Metalloborospherenes: M@B40 (M=Ca, Sr) and M&B40 (M=Be, Mg). Angew. Chem. Int. Ed. 54:941–945. https://doi.org/10.1002/anie.201408738
Li HR, Liu H, Tian XX, Zan WY, Mu YW, Lu HG, Li J, Wang YK, Li SD (2017) Structural transition in metal-centered boron clusters: from tubular molecular rotors Ta@B21 and Ta@B22+ to cage-like endohedral metalloborospherene Ta@B22−. Phys. Chem. Chem. Phys. 19:27025–27030. https://doi.org/10.1039/C7CP05179D
Yu TR, Gao Y, Xu DX, Wang ZG (2018) Actinide endohedral boron clusters: a closed-shell electronic structure of U@B40. Nano Res. 11:354. https://doi.org/10.1007/s12274-017-1637-9
Oger E, Crawford NRM, Kelting R, Weis P, Kappes MM, Ahlrichs R (2007) Boron cluster Cations: transition from planar to cylindrical structures. Angew. Chem. Int. Ed. 46:8503–8506. https://doi.org/10.1002/anie.200701915
Sai LW, Wu X, Gao N, Zhao JJ, King RB (2017) Boron clusters with 46, 48, and 50 atoms: competition among the core–shell, bilayer and quasi-planar structures. Nanoscale 9:13905–13909. https://doi.org/10.1039/C7NR02399E
Pei L, Ma YY, Yan M, Zhang M, Yuan RN, Chen Q, Zan WY, Mu YW, Li SD (2020) Bilayer B54, B60, and B62 clusters in a universal structural pattern. Eur. J. Inorg. Chem. 34:3296–3301. https://doi.org/10.1002/ejic.202000473
Romanescu C, Galeev TR, Li WL, Boldyrev AI, Wang LS (2011) Aromatic metal-centered monocyclic boron rings: Co©B8− and Ru©B9−. Angew. Chem. Int. Ed. 50:9334–9337. https://doi.org/10.1002/ange.201104166
Galeev TR, Romanescu C, Li WL, Wang LS, Boldyrev AI (2012) Observation of the highest coordination number in planar species: Decacoordinated Ta©B10− and Nb©B10− anions. Angew. Chem. Int. Ed. 51:2101–2105. https://doi.org/10.1002/ange.201107880
Jian T, Li WL, Popov IA, Lopez GV, Chen X, Boldyrev AI, Li J, Wang LS (2016) Manganese-centered tubular boron cluster – MnB16−: a new class of transition-metal molecules. J. Chem. Phys. 144:154310. https://doi.org/10.1063/1.4946796
Popov IA, Jian T, Lopez GV, Boldyrev AI, Wang LS (2015) Cobalt-centred boron molecular drums with the highest coordination number in the CoB16− cluster. Nat. Commun. 6:8654. https://doi.org/10.1038/ncomms9654
Jian T, Li WL, Chen X, Chen TT, Lopez GV, Li J, Wang LS (2016) Competition between drum and quasi-planar structures in RhB18−: motifs for metallo-boronanotubes and metallo-borophenes. Chem. Sci. 7:7020–7027. https://doi.org/10.1039/C6SC02623K
Li WL, Jian T, Chen X, Li HR, Chen TT, Luo XM, Li SD, Li J, Wang LS (2017) Observation of a metal-centered B2-Ta@B18− tubular molecular rotor and a perfect Ta@B20− boron drum with the record coordination number of twenty. Chem. Commun. 53:1587–1590. https://doi.org/10.1039/C6CC09570D
Li WL, Chen TT, Xing DH, Chen X, Li J, Wang LS (2018) Observation of highly stable and symmetric lanthanide octa-boron inverse sandwich complexes. Proc. Natl. Acad. Sci. U. S. A. 115:E6972–E6977. https://doi.org/10.1073/pnas.1806476115
Chen TT, Li WL, Li J, Wang LS (2019) [La(ηx-Bx)La]− (x = 7–9): a new class of inverse sandwich complexes. Chem. Sci. 10:2534–2542. https://doi.org/10.1039/c8sc05443f
Chen TT, Li WL, Chen WJ, Li J, Wang LS (2019) La3B14−: an inverse triple-decker lanthanide boron cluster. Chem. Commun. 55:7864–7867. https://doi.org/10.1039/c9cc03807h
Chen TT, Li WL, Chen WJ, Yu XH, Dong XR, Li J, Wang LS (2020) Spherical trihedral metallo-borospherenes. Nat. Commun. 11:2766. https://doi.org/10.1038/s41467-020-16532-x
Lu XQ, Chen Q, Tian XX, Mu YW, Lu HG, Li SD (2019) Predicting lanthanide boride inverse sandwich tubular molecular rotors with the smallest core–shell structure. Nanoscale 11:21311–21316. https://doi.org/10.1039/c9nr07284e
Zhao XY, Yan M, Wei ZH, Li SD (2020) Donor–acceptor duality of the transition-metal-like B2 core in core–shell-like metallo-borospherenes La3&[B2@B17]− and La3&[B2@B18]−. RSC Adv. 10:34225–34230. https://doi.org/10.1039/d0ra06769e
Zhang Y, Zhao XY, Yan M, Li SD (2020) From inverse sandwich Ta2B7+ and Ta2B8 to spherical trihedral Ta3B12−: prediction of the smallest metallo-borospherene. RSC Adv. 10:29320–29325. https://doi.org/10.1039/d0ra05570k
Gao Y, Zeng XC (2005) M4@Si28 (M=Al, Ga): metal-encapsulated tetrahedral silicon fullerene. J. Chem. Phys. 123:204325. https://doi.org/10.1063/1.2121568
Guo T, Diener Chai M, Alford MJ, Haufler RE, McClure SM, Ohno T, Weaver JH, Scuseria GE, Smalley RE (1992) Science Uranium Stabilization of C28 : A Tetravalent Fullerene. 257: 1661–1664. https://doi.org/10.1126/science.257.5077.1661
Chen X, Zhao YF, Zhang YY, Li J (2019) TGMin: an efficient global minimum searching program for free and surface-supported clusters. J. Comput. Chem. 40:1105–1112. https://doi.org/10.1002/jcc.25649
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110:6158–6170. https://doi.org/10.1063/1.478522
Tao J, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: non-empirical meta-generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 91:146401. https://doi.org/10.1103/PhysRevLett.91.146401
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72: 650. https://doi.org/10.1063/1.438955
Feller D (1996) The role of databases in support of computational chemistry calculations. J. Comput. Chem. 17:1571–1586. https://doi.org/10.1002/(SICI)1096-987X
Schuchardt L, Didier B, Elsethagen T, Sun L, Gurumoorthi V, Chase J, Li J, Windus TL (2007) Basis set exchange: a community database for computational sciences. J. Chem. Inf. Model. 47:1045–1052. https://doi.org/10.1021/ci600510j
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2009) Gaussian 09, revision D.01. Gaussian, Inc., Wallingford
Čížek J (1969) On the use of the cluster expansion and the technique of diagrams in calculations of correlation effects in atoms and molecules. Adv. Chem. Phys. 14:35–89. https://doi.org/10.1002/9780470143599.ch2
Purvis III GD, Bartlett RJ (1982) A full coupled-cluster singles and doubles model: the inclusion of disconnected triples. J. Chem. Phys. 76:1910. https://doi.org/10.1063/1.443164
Raghavachari K, Trucks GW, Pople JA, Head-Gordon M (1989) A fifth-order perturbation comparison of electron correlation theories. Chem Phys Lett 157: 479-483. Https:// doi.org/157.1989/479
Werner HJ, et al., Molpro, version 2012.1
Tkachenko NV, Boldyrev AI (2019) Chemical bonding analysis of excited states using the adaptive natural density partitioning method. Phys. Chem. Chem. Phys. 21:9590–9596. https://doi.org/10.1039/c9cp00379g
Glendening PED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F. NBO 6.0, 2013
VandeVondele J, Krack M, Mohamed F, Parrinello M, Chassaing T, Hutter J (2005) QUICKSTEP: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167:103–128. https://doi.org/10.1016/j.cpc.2004.12.014
Alexandrova AN, Birch KA, Boldyrev AI (2003) Flattening the B6H62− octahedron Ab initio prediction of a new family of planar all-boron aromatic molecules. J. Am. Chem. Soc. 125:10786–10787. https://doi.org/10.1021/ja0361906
Schleyer PR, Maerker C (1996) Nucleus-independent chemical shifts: a simple and efficient Aromaticity probe. J. Am. Chem. Soc. 118:6317–6318. https://doi.org/10.1021/JA960582D
Ciuparu D, Klie R, Zhu YM, Pfefferle L (2004) Synthesis of pure boron Single-Wall nanotubes. J. Phys. Chem. B 108:3967–3969. https://doi.org/10.1021/jp049301b
Availability of data and material
All the data are available online.
Code availability
N/A
Funding
The work was supported by the National Natural Science Foundation of China (21720102006 and 21973057 to S.-D. Li).
Author information
Authors and Affiliations
Contributions
Z. H. Wei and S. D. Li designed the project and X. Q. Lu and C. Y. Gao performed the calculations. All the authors participate in the discussion and preparation of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1.99 mb)
Rights and permissions
About this article
Cite this article
Lu, XQ., Gao, CY., Wei, Z. et al. Cage-like La4B24 and Core-Shell La4B290/+/− : perfect spherically aromatic tetrahedral metallo-borospherenes. J Mol Model 27, 130 (2021). https://doi.org/10.1007/s00894-021-04739-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-021-04739-8