Abstract
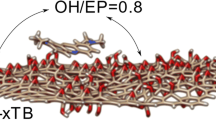
A density functional theory augmented by the long-range corrected hybrid density functional ωB97XD and 6-31G(d,p) basis set has been applied to generate sandwich structures consist of nanocomposites between graphene oxide and polyvinyl alcohol. We predicted the interaction energies and discuss the contribution of electrostatic and dispersion components. Also, we computationally generated IR spectra of intercalates and compared them with those experimentally obtained. Two sources of interaction energy to stabilize the intercalates between graphene oxide and PVA are suggested. They are the electrostatic and dispersion (van-der-Waals) components. We also revealed that ωB97XD density functional in conjunction with 6-31G(d,p) basis set is qualitatively able to describe IR spectra of considered species.



Similar content being viewed by others
References
Sun Y, Lu J, Ai C, Wen D (2016) Nonvolatile memory devices based on poly(vinyl alcohol) + graphene oxide hybrid composites. Phys Chem Chem Phys 18:11341–11347. https://doi.org/10.1039/C6CP00007J
Duran N, Martinez D, Silveira C et al (2015) Graphene oxide: a carrier for pharmaceuticals and a scaffold for cell interactions. Curr Top Med Chem 15:309–327. https://doi.org/10.2174/1568026615666150108144217
Ahmed J (2019) Use of graphene/graphene oxide in food packaging materials: thermomechanical, structural and barrier properties. Reference Module in Food Science. Elsevier, In, pp 219–245
El Miri N, El Achaby M, Fihri A et al (2016) Synergistic effect of cellulose nanocrystals/graphene oxide nanosheets as functional hybrid nanofiller for enhancing properties of PVA nanocomposites. Carbohydr Polym 137:239–248. https://doi.org/10.1016/j.carbpol.2015.10.072
Shen H, Zhang L, Liu M, Zhang Z (2012) Biomedical applications of graphene. Theranostics 2:283–294. https://doi.org/10.7150/thno.3642
Down MP, Rowley-Neale SJ, Smith GC, Banks CE (2018) Fabrication of graphene oxide supercapacitor devices. ACS Appl Energy Mater 1:707–714. https://doi.org/10.1021/acsaem.7b00164
Da Chai J, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620. https://doi.org/10.1039/b810189b
Mardirossian N, Head-gordon M (2017) Thirty years of density functional theory in computational chemistry : an overview and extensive assessment of 200 density functionals. 8976:. https://doi.org/10.1080/00268976.2017.1333644
Leonid Gorb et al. Effect of micro-environment on the geometrical structure of d(a)5 d(T)5, d(G)5d (C)5 DNA mini-helixes and Dickerson Dodecamer: a density functional theory study. Prog
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
G. Schaftenaar Molden a pre- and post processing program of molecular and electronic structure
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. and DJF Gaussian 16, Revision C.01
PyMOL The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC
Politzer P, Truhlar DG (1981) Chemical applications of atomic and molecular electrostatic potentials. Springer US, Boston, MA
Chen W, Gordon MS (1996) Energy decomposition analyses for many-body interaction and applications to water complexes. J Phys Chem 100:14316–14328. https://doi.org/10.1021/jp960694r
Glendening ED (1996) Natural energy decomposition analysis: explicit evaluation of electrostatic and polarization effects with application to aqueous clusters of alkali metal cations and neutrals. J Am Chem Soc 118:2473–2482. https://doi.org/10.1021/ja951834y
M.V. Karachevtsev, S.G. Stepanian, L. Adamowicz VAK Modeling of nucleobase/oligonucleotide interaction with graphene oxide/graphene: the role of oxidation degree and charged surface of graphene. Mol Cryst Liq Cryst
Clark T, Murray JS, Politzer P (2018) A perspective on quantum mechanics and chemical concepts in describing noncovalent interactions. Phys Chem Chem Phys 20:30076–30082. https://doi.org/10.1039/c8cp06786d
Politzer P, Murray JS, Clark T (2015) Mathematical modeling and physical reality in noncovalent interactions. J Mol Model 21. https://doi.org/10.1007/s00894-015-2585-5
Wang T, Li Y, Geng S et al (2015) Preparation of flexible reduced graphene oxide/poly(vinyl alcohol) film with superior microwave absorption properties. RSC Adv 5:88958–88964. https://doi.org/10.1039/c5ra16158d
Andrijanto E, Shoelarta S, Subiyanto G (1725) Rifki S (2016) Facile synthesis of graphene from graphite using ascorbic acid as reducing agent. AIP Conf Proc. https://doi.org/10.1063/1.4945457
Zamani M, Gholibegloo E, Aghajanzadeh M et al (2020) Polyvinyl alcohol-graphene oxide nanocomposites: evaluation of flame-retardancy, thermal and mechanical properties. J Macromol Sci Part A Pure Appl Chem 57:17–24. https://doi.org/10.1080/10601325.2019.1578617
Funding
This study was supported by the NSF PREM grant #1826886.
The computation time was provided by the Extreme Science and Engineering Discovery Environment (XSEDE) by National Science Foundation Grant Number OCI-1053575 and XSEDE award allocation Number DMR110088 and by the Mississippi Center for Supercomputer Research.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 559 kb)
Rights and permissions
About this article
Cite this article
Gorb, L., Ilchenko, M. & Leszczynski, J. A density functional theory study of simplest nanocomposites formed by graphene oxide and polyvinyl alcohol: geometry, interaction energy and vibrational spectrum. J Mol Model 26, 183 (2020). https://doi.org/10.1007/s00894-020-04447-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04447-9