Abstract
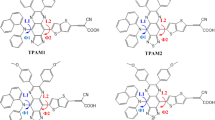
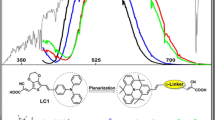
On the basis of triphenylamine as an electron donor with attachment of two –COOH anchoring groups and dicyanovinyl as acceptor, ten dyes with D-π-A structures were designed to investigate the effects of different π-linker groups on the properties of the sensitizers, especially the influence of the π-linkers containing nitrogen cation (N+). The optimized structures and electronic and optical properties were investigated by the density functional theory (DFT) and time-dependent DFT (TD-DFT). The results show that all the investigated dyes can be used as dye sensitizers for the p-type dye-sensitized solar cells (DSSCs) except one dye which contains two N+. The N+ modified dye (named S3-PZL1C) has narrow energy gap (2.02 eV), the best light-harvesting efficiency (LHE, 0.9974), and the smallest internal reorganization energy (λint = 7.00 kcal/mol). Importantly, S3-PZL1C displays the largest red shift of the UV-vis absorption, the maximum integral values of the adsorption-wavelength curves over the visible light (400~800 nm), and the strongest adsorption energy (− 66.84 kcal/mol) on NiO surface. In addition, S3-PZL1C not only enhances the electronic excitation but also improves the reorganization energy and charge separation. The intramolecular charge transfer towards the acceptor is sensitive to the N+ position in π-linkers. Therefore, the suitable introduction of N+ in dyes can improve the performance of the dyes, and the PZL1C moiety may be a promising π-linker for p-type DSSCs.

Graphical abstract









Similar content being viewed by others
References
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Shockley W, Queisser HJ (1961) Slip patterns on boron-doped silicon surfaces. J Appl Phys 32:1776
Bhattacharya S, John S (2018) Designing high-efficiency thin silicon solar cells using parabolic-pore photonic crystals. Phys Rev Appl 9:044009
Li X, Yu F, Stappert S, Li C, Zhou Y, Yu Y, Li X, Ågren H, Hua J, Tian H (2018) Enhanced photocurrent density by spin-coated NiO photocathodes for N-annulated perylene-based p-type dye-sensitized solar cells. ACS Appl Mater Interfaces 8:19393–19401
Ji Z, Natu G, Huang Z, Wu Y (2011) Linker effect in organic donor–acceptor dyes for p-type NiO dye sensitized solar cells. Energy Environ Sci 4:2818–2821
Qin P, Linder M, Brinck T, Boschloo G, Hagfeldt A, Sun L (2009) High incident photon-to-current conversion efficiency of p-type dye-sensitized solar cells based on NiO and organic chromophores. Adv Mater 21:2993–2996
Qin P, Zhu H, Edvinsson T, Boschloo G, Hagfeldt A, Sun L (2008) Design of an organic chromophore for p-type dye-sensitized solar cells. J Am Chem Soc 130:8570–8571
Zhang G, Bai Y, Li R, Shi D, Wenger S, Zakeeruffin SM, Grätzel M, Wang P (2009) Employ a bisthienothiophene linker to construct an organic chromophore for efficient and stable dye-sensitized solar cells. Energy Environ Sci 2:92–95
Liu W, Wu I, Lai C, Lai C, Chou P, Li Y, Chen C, Hsu Y, Chi Y (2008) Simple organic molecules bearing a 3,4-ethylenedioxythiophene linker for efficient dye-sensitized solar cells. Aust J Chem 40:5152–5154
Li R, Lv X, Dong S, Zhou D, Cheng Y, Zhang G, Wang P (2009) Dye-sensitized solar cells based on organic sensitizers with different conjugated linkers: furan, bifuran, thiophene, bithiophene, selenophene, and biselenophene. J Phys Chem C 113(17):7469–7479
Nattestad A, Mozer AJ, Fischer MKR, Cheng YB, Mishra A, Bäuerle P, Bach U (2010) Highly efficient photocathodes for dye-sensitized tandem solar cells. Nat Mater 9:31–35
Shin W, Kim S, Lee S, Jeon H, Kim M, Naidu B, Jin S, Lee J, Lee J, Gal Y (2010) Synthesis and photovoltaic properties of a low-band-gap polymer consisting of alternating thiophene and benzothiadiazole derivatives for bulk-heterojunction and dye-sensitized solar cells. J Polym Sci A Polym Chem 45:1394–1402
Jung I, Yu J, Jeong E, Kim J, Kwon S, Kong H, Lee K, Woo H, Shim H (2010) Synthesis and photovoltaic properties of cyclopentadithiophene-based low-bandgap copolymers that contain electron-withdrawing thiazole derivatives. Chem Eur J 16:3743–3752
Li H, Zhang J, Wu Y, Jin J, Duan Y, Su Z, Geng Y (2014) Theoretical study and design of triphenylamine-malononitrile-based p-type organic dyes with different π-linkers for dyes-sensitized solar cells. Dyes Pigments 108:106–114
Bo H, Freeman H (2013) New N-methyl pyrrole and thiophene based D-π-A systems for dye-sensitized solar cells. Dyes Pigments 96:313–318
Zhang F, Yu P, Shen W, Li M, He R (2016) Comparison of p-type sensitizers with different electron-induced effects in dye-sensitized solar cells: a theoretical investigation. Comput Theor Chem 1095:118–124
Langmar O, Saccone D, Amat A, Fantacci S, Viscardi G, Barolo C, Costa R, Guldi DM (2017) Designing squaraines to control charge injection and recombination processes in NiO-based dye-sensitized solar cells. ChemSusChem 10:2385–2393
Qi X, Fei W, Han M, Zhen L, Zhu L, Li Z (2018) A pseudo-two-dimensional conjugated polysquaraine: an efficient p-type polymer semiconductor for organic photovoltaics and perovskite solar cells. J Mater Chem A 6:13644–13651
Bonomo M, Barbero N, Matteocci F, Carlo AD, Barolo C, Dini D (2016) Beneficial effect of electron-withdrawing groups on the sensitizing action of squaraines for p-type dye-sensitized solar cells. J Phys Chem C 120:16340–16353
Yong S, Yang Y, Kim J, Ryu J, Kim K, Park S (2010) Organic photosensitizers based on terthiophene with alkyl chain and double acceptors for application in dye-sensitized solar cells. Energy Fuel 24:3676–3681
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR et al (2009) Gaussian 09W, revision D.01. Gaussian, Inc., Wallingford
Xia H, Wang J, Bai F, Zhang H, Xia H, Wang J, Bai F, Zhang HX (2015) Theoretical studies of electronic and optical properties of the triphenylamine-based organic dyes with diketopyrrolopyrrole chromophore. Dyes Pigments 113:87–95
Warnan J, Favereau L, Pellegrin Y, Blart E, Jacquemin D, Odobel F (2011) A compact diketopyrrolopyrrole dye as efficient sensitizer in titanium dioxide dye-sensitized solar cells. J Photoch Photobio A 226:9–15
Obotowo IN, Obot IB, Ekpe UJ (2016) Organic sensitizers for dye-sensitized solar cell (DSSC): properties from computation, progress and future perspectives. J Mol Struct 1122:80–87
Keith TA, Bader RFW (1992) Calculation of magnetic response properties using atoms in molecules. Chem Phys Lett 194:1–8
Herges R, Geuenich D (2001) Delocalization of electrons in molecules. J Phys Chem C 105:3214–3220
Li J, Zhang S, Shao D, Yang Z, Zhang W (2018) Effect of auxiliary group for p-type organic dyes in NiO-based dye-sensitized solar cells: the first principal study. Spectrochim Acta A 193:192–196
Zheng Y, Sun S, Xu L, Ni S, Liu W, Huang B, Huang Q, Zhang Q, Lu F, Li M (2019) Arylamine-coumarin based donor-acceptor dyads: unveiling the relationship between two-photon absorption cross-section and lifetime of singlet excited state intramolecular charge separation. Dyes Pigments 165:301–307
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Odobel F, Pellegrin Y (2013) Recent advances in the sensitization of wide-band-gap nanostructured p-type semiconductors. Photovoltaic and photocatalytic applications. J Phys Chem Lett 4:2551–2564
Li L, Gibson EA, Qin P, Boschloo G, Gorlov M, Hagfeldt A, Sun L (2010) Double-layered NiO photocathodes for p-type DSSCs with record IPCE. Adv Mater 22:1759–1762
Sun L, Zhang T, Wang J, Li H, Yan L, Su ZM (2015) Exploring the influence of electron donating/withdrawing groups on hexamolybdate-based derivatives for efficient p-type dye-sensitized solar cells (DSSCs). RSC Adv 5:39821–39827
Zhang F, Yu P, Shen W, Li M, He R (2015) Effect of “push–pull” sensitizers with modified conjugation bridges on the performance of p-type dye-sensitized solar cells. RSC Adv 5:64378–64386
Assyry AE, Hallaoui A, Saddik R, Benchat N, Benali B, Zarrouk A (2015) Gaussian electronic properties studies of organic materials based on pyridazine for efficient photovoltaic devices. Der Pharmacia Lett 7:295–304
Chitpakdee C, Namuangruk S, Suttisintong K, Jungsuttiwong S, Keawin T, Sudyoadsuk T, Sirithip K, Promarak V, Kungwan N (2015) Effects of π-linker, anchoring group and capped carbazole at meso-substituted zinc-porphyrins on conversion efficiency of DSSCs. Dyes Pigments 118:64–75
Geuenich D, Hess K, Köhler F, Herges R (2005) Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem Rev 105:3758–3772
Montero-Campillo MM, Rodríguez-Otero J, Cabaleiro-Lago EM (2007) Ab initio and DFT study of the aromaticity of some fulvalenes derived from methylidenecyclopropabenzene. J Mol Model 13:919–926
Suresh T, Chitumalla RK, Hai N, Jang J, Lee T, Kim J (2016) Impact of neutral and anion anchoring groups on the photovoltaic performance of triphenylamine sensitizers for dye-sensitized solar cells. RSC Adv 6:26559–26567
Odobel F, Pellegrin Y, Gibson EA, Hagfeldt A, Smeigh AL, Hammarström L (2012) Recent advances and future directions to optimize the performances of p-type dye-sensitized solar cells. Coord Chem Rev 256:2414–2423
Zhang J, Li H, Sun S, Geng Y, Wu Y, Su Z (2011) Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J Mater Chem 22:568–576
Bao LQ, Ho P, Chitumalla RK, Jang J, Thogiti S, Kim JH (2018) Single and double branched organic dyes based on carbazole and red-absorbing cationic indolium for p-type dye-sensitized solar cells: a combined experimental and theoretical investigation. Dyes Pigments 149:25–36
Ma W, Jiao Y, Meng S (2014) Predicting energy conversion efficiency of dye solar cells from first principles. J Phys Chem C 118:16447–16457
Odobel F, Pleux LL, Pellegrin Y, Blart E (2010) New photovoltaic devices based on the sensitization of p-type semiconductors: challenges and opportunities. Acc Chem Res 43:1063–1071
Borgström M, Blart E, Boschloo G, Mukhtar E, Hagfeldt A, Hammarström L, Odobel F (2015) Sensitized hole injection of phosphorus porphyrin into NiO: toward new photovoltaic devices. J Phys Chem B 109:22928
Morandeira A, Boschloo G, Hagfeldt A, Hammarström L (2005) Photoinduced ultrafast dynamics of coumarin 343 sensitized p-type-nanostructured NiO films. J Phys Chem B 109:19403–19410
Yamada T, Sato T, Tanaka K, Kaji H (2010) Percolation paths for charge transports in N,N′-diphenyl-N,N′-di(m-tolyl)benzidine (TPD). Org Electron 11:255–265
Vittadini A, Selloni A, Rotzinger FP, Grätzel M (2000) Formic acid adsorption on dry and hydrated TiO2 anatase (101) surfaces by DFT calculations. J Phys Chem B 104:1300–1306
Nazeeruddin MK, Humphry-Baker R, Liska P, Graetzel MJJ (2003) Investigation of sensitizer adsorption and the influence of protons on current and voltage of a dye-sensitized nanocrystalline TiO2 solar cell. J Phys Chem B 107:8981–8987
Surratt GT, Kunz AB (1979) Theoretical study of H chemisorption on NiO. Perfect surfaces and cation vacancies. Phys Rev B 19:2352–2358
Funding
This work was partially supported by the Research Innovation Program for College Graduates of Jiangsu Province (KYLX16-0468) and the project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 766 kb)
Rights and permissions
About this article
Cite this article
Sun, ZD., Zhao, JS., Mei, Z. et al. Theoretical study of nitrogen cation modified aromatics containing thiophene as π-linker for p-type photosensitizers. J Mol Model 25, 300 (2019). https://doi.org/10.1007/s00894-019-4179-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4179-0