Abstract
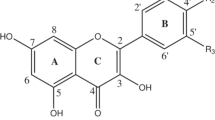
Glycosylated flavonoids are often found in supplementary food sources and are structurally more active than aglycones, as the glycosyl unit offers better solubility, stability, and functionality. The most common glycosyl units present in most secondary metabolites are glucoside and rhamnoside attached to flavonoid moiety. In the present work, 4’-O–glycosylated (4’-O-glucoside and 4’-O-rhamnoside) demethyltexasin, a soy isoflavone bearing a chromen nucleus, is investigated through a green route aided by density functional theory. Antioxidant activity is computed via three different antioxidant mechanisms, in which hydrogen atom abstraction is facilitated effectively in both gas and solvent phases. The 6–OH site (A-ring) in both isoflavone demethyltexasin glucoside (DMTG) and isoflavone demethyltexasin rhamnoside (DMTR) acted as better radical scavenging sites. Charge localization in B-ring is well altered by the glycosyl and rhamnosyl groups attached. Target prediction analysis supports DMTG with good binding ability with the selected homosapien class targets. The results depict that even though the flavonoid possessing rhamnosyl unit induces charge delocalization at the 4’-O position, it offers resistance toward antioxidant activity when compared to glycosyl moeity.

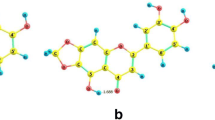
The contour representation of antioxidants demethyltexasin 4’-O- glucoside (DMTG) and demethyltexasin 4’-O- rhamnoside (DMTR) against reactive oxygen species(ROS)





Similar content being viewed by others
References
Nakamura Y, Tsuji S, Tonogai Y (2000) Determination of the levels of isoflavonoids in soybeans and soy–derived foods and estimation of isoflavonoids in the Japanese daily diet. J AOAC Int 83:635–650
Klejdus B, Vitamvasova–Sterbova D, Kuban V (2001) Identification of isoflavone conjugates in red clover (Trifolium pratense) by liquid chromatography–mass spectrometry after two–dimensional solid–phase extraction. Anal Chim Acta 450:81–97
Chang YC, Nair MG (1995) Metabolism of daidzein and genistein by intestinal bacteria. J Nat Prod 58:1892–1896
Chung HL, Yang L, Xu JZ, Yeung SYV, Huang Y, Chen ZY (2005) Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem 90:735–741
Meghwal M, Sahu CK (2015) Soy isoflavonoids as nutraceutical for human health: an update. J Cell Sci Ther 6:194. https://doi.org/10.4172/2157-7013.1000194
Galano A, Mazzone G, Alvarez-Diduk R, Marino T, Alvarez-Idaboy JR, Russo N (2016) Food antioxidants: chemical insights at the molecular level. Annu Rev Food Sci Technol 7:15.1–15.18
Lilienfeld V, Anatole O, Tavernelli I, Rothlisberger U, Sebastiani D (2004) Optimization of effective atom centered potentials for London dispersion forces in density functional theory. Phys Rev Lett 93(15):153004
Hummelova J, Rondevaldova J, Balastikova A, Lapcikand O, Kokoska L (2014) The relationship between structure and in vitro antibacterial activity of selected isoflavones and their metabolites with special focus on anti-staphylococcal effect of demethyltexasin. Lett Appl Microbiol 60:242–247
Harborne (1999) Isoflavonoids and neoflavonoids. The handbook of natural flavonoids, vol 2. Wiley, New York, p 517
Friedlander A, Sklarz B (1971) Structural elucidation of isoflavones from Glycine max, Experientia 27:762
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision D.01. Gaussian Inc., Wallingford
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717
Deepha V, Praveena R, Sadasivam K (2015). J Mol Struct 1082:131e142
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H–atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922
Leopoldini M, Prieto Pitarch I, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem B 108:92–94
Rüfer CE, Kulling SE (2006) Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem 54:2926–2931
Jovanovic SV, Hara Y, Steenken S, Simic MG (1995) Antioxidant potential of gallocatechins. A pulse radiolysis and laser photolysis study. J Am Chem Soc 117:9881–9888
Apak R, Güçlü K, Demirata B, Özyürek M, Çelik SE, Bektaşoğlu B, Berker KI, Özyurt D (2007) Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 12:1496–1547
Han R–M, Tian Y–X, Becker EM, Andersen ML, Zhang J–P, Skibsted LH (2007) Puerarin and conjugate bases as radical scavengers and antioxidants: molecular mechanism and synergism with β–carotene. J Agric Food Chem 55:2384–2391
Mendoza–Wilson AM, Lardizabal–Gutierrez D, Torres–Moye E, Fuentes–Cobas L, Balandran–Quintana RR, Camacho–Davila A, Quintero–Ramos A, Glossman–Mitnik D (2007) Optimized structure and thermochemical properties of flavonoids determined by the CHIH(medium)–DFT model chemistry versus experimental techniques. J Mol Struct 871:114–130
Zheng YZ, Deng G, Liang Q, Chen DF, Guo R, Lai RC (2017) Antioxidant activity of quercetin and its glucosides from propolis: a theoretical study. Sci Rep 7(1):7543. https://doi.org/10.1038/s41598-017-08024-8.
Estevez L, Otero N, Mosquera RA (2010) A computational study on the acidity dependence of radical–scavenging mechanisms of anthocyanidins. J Phys Chem B 114:9706–9712
JR L–C, JR A–I, Galano A (2012) On the peroxyl scavenging activity of hydroxycinnamic acid derivatives: mechanisms, kinetics, and importance of the acid–base equilibrium. Phys Chem Chem Phys 14:12534–12543 32
Trouilla P, Marsal P, Siri D, Lazzaroni R, Duroux J-L (2006) A DFT study of the reactivity of OH groups in quercetin and taxifolin antioxidants: the specificity of the 3-OH site. Food Chem 97(4):679–688 ISSN: 0308-8146
Rong Y, Wang Z, Wu J, Zhao B (2012) A theoretical study on cellular antioxidant activity of selected flavonoids. Spectrochim Acta A 93:235–239
Manz TA, Gabaldon–Limas N (2016) Introducing DDEC6 atomic population analysis: part 1. Charge partitioning theory and methodology. RSC Adv 6:47771–47801. https://doi.org/10.1039/c6ra04656h
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Lu T, Chen F (2011) Calculation of molecular orbital composition. Acta Chim Sin 69:2393–2406
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comp Chem 33:580–592
Bader RFW, Johnson S, Tang TH, Popelier PLA (1996). J Phys Chem 100:15398
Frasca M (1998) Duality in perturbation theory and the quantum adiabatic approximation. Phys Rev A 58(5):3439
Sadlej-Sosnowska N (2001) Application of natural bond orbital analysis to delocalization and aromaticity in C-substituted tetrazoles. J Org Chem 66:8737–8743
Gfeller D, Grosdidier A, Wirth M, Daina A, Michielin O, Zoete V (2014) SwissTargetPrediction: a web server for target prediction of bioactive small molecules. Nucleic Acids Res 42(Web Server issue):W32–W38
Perez–Miller SJ, Hurley TD (2003) Coenzyme isomerization is integral to catalysis in aldehyde dehydrogenase. Biochemistry 42(23):7100–7109
Krupenko NI, Dubard ME, Strickland KC, Moxley KM, Oleinik NV, Krupenko SA (2010) ALDH1L2 is the mitochondrial homolog of 10–formyltetrahydrofolate dehydrogenase. J Biol Chem 285(30):23056–23063
Acknowledgments
The authors are sincerely thankful to the Science and Engineering Research Board, Department of Science and Technology (DST–SERB), and Government of India for funding the research. Grant Id. (EMR/2016/002892).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors state that there is not any potential conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 45 kb)
Rights and permissions
About this article
Cite this article
Anbazhakan, K., Sadasivam, K., Praveena, R. et al. Target prediction and antioxidant analysis on isoflavones of demethyltexasin: a DFT study. J Mol Model 25, 169 (2019). https://doi.org/10.1007/s00894-019-4045-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-019-4045-0