Abstract
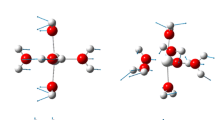
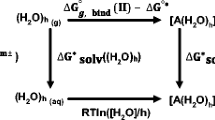
Thermodynamic and structural aspects of the hydration of Pb(II) ions were explored based on DFT calculations combined with the supermolecular/continuum solvent model. Hydration of Pb(II) was considered as the formation of Pb(H2O)n2+ aqua complexes (n=6−9) from the gas phase Pb(II) ion. Hexa- and hepta-aqua Pb(II) complexes were shown to exhibit the hemidirected symmetry, while those containing eight and nine water molecules are characterized by the holodirected symmetry. The calculations showed that because Pb(H2O)n2+ complexes with six to nine water molecules have comparable thermodynamic stabilities, such complexes are likely to coexist in aqueous solutions. The deprotonation of Pb(H2O)n2+ complexes was shown to result in the formation of the mono-hydroxo complex [Pb(H2O)4OH]+. The pKa1 value determined for this reaction (7.58 for Pb(H2O)62+) was close to the experimental value of 7.61 used in recent models of aquatic equilibria. The density functional method ω-B97X(PCM-UAO) in combination with the atomic basis set 6-311++G(d,p) for O and H and the small-core electron effective pseudopotential (ECP) with the aug-cc-pvdz-PP basis set for Pb can be recommended for such calculations.

Structures of Pb(II) ions with varying numbers of water molecules in the inner hydration shell


Similar content being viewed by others
References
Marcus Y (1985) Ion solvation. Wiley, Chichester
Ben-Naim A (1987) Solvation thermodynamics. Plenum, New York
Bryantsev VS, Diallo MS, Godard III WA (2008) Calculation of solvation free energies of charged solutes using mixed cluster/continuum models. J Phys Chem B 112:9709–9719. https://doi.org/10.1021/jp802665d
Brown MJ, Raymond J, Homa D, Kennedy C, Sinks T (2011) Association between children’s blood lead levels, lead service lines, and water disinfection, Washington, DC, 1998−2006. Environ Res 111:67–74. https://doi.org/10.1016/j.amjmed.2016.05.042
Triantafyllidou S, Edwards M (2012) Lead (Pb) in tap water and in blood: implications for lead exposure in the United States. Rev Environ Sci Technol 42:1297–1352. https://doi.org/10.1080/10643389.2011.556556
Liu H, Kuznetsov AM, Masliy AN, Ferguson JF, Korshin GV (2012) Formation of Pb(III) intermediates in the electrochemically controlled Pb(II)/PbO2 system. Environ Sci Technol 46:1430–1438. https://doi.org/10.1021/es203084n
Lytle DA, Schock MR (2005) Formation of Pb(IV) oxides in chlorinated water. J Am Water Works Assoc 97:102–114. https://doi.org/10.1021/es102318z
Noel JD, Wang Y, Giammar DE (2014) Effect of water chemistry on the dissolution rate of the lead corrosion product hydrocerussite. Water Res 54:237–246. https://doi.org/10.1016/j.watres.2014.02.004
Edwards M, Triantafyllidou S (2007) Chloride-to-sulfate mass ratio and lead leaching to water. J Am Water Works Assoc 99:96–109
Xie Y, Giammar DE (2011) Effects of flow and water chemistry on lead release rates from pipe scales. Water Res 45:6525–6534. https://doi.org/10.1016/j.watres.2011.09.050
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JJA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B.01. Gaussian Inc., Wallingford
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parameterization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Chai J-D, Head-Gordon M (2008a) Systematic optimization of long-range corrected hybrid density functionals. J Chem Phys 128:084106–084115. https://doi.org/10.1063/1.2834918
Chai J-D, Head-Gordon M (2008b) Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys Chem Chem Phys 44:6615–6620. https://doi.org/10.1039/B810189B
Zhao Y, Truhlar DG (2006) A new local density functional for main-group thermochemistry, transition metal bonding, thermochemical kinetics, and noncovalent interactions. J Chem Phys 125:194101: 1–18. https://doi.org/10.1063/1.2370993
Tao JM, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401. https://doi.org/10.1103/PhysRevLett.91.146401
Staroverov VN, Scuseria GE, Tao J, Perdew JP (2003) Comparative assessment of a new nonempirical density functional: molecules and hydrogen-bonded complexes. J Chem Phys 119:12129. https://doi.org/10.1063/1.1626543. [Erratum] 121 (2004) 11507(E). doi:10.1063/1.1795692
Dunning Jr TH (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–−1023. https://doi.org/10.1063/1.456153
Peterson KA (2003) Systematically convergent basis sets with relativistic pseudopotentials. I. Correlation consistent basis sets for the post-d group 13–15 elements. Chem Phys 119:11099–11112. https://doi.org/10.1063/1.1622923
Metz B, Stoll H, Dolg M (2000) Small-core multiconfiguration Dirac-Hartree-Fock-adjusted pseudopotentials for post-d main group elements: application to PbH and PbO. J Chem Phys 113:2563–2569. https://doi.org/10.1063/1.1305880
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Persson I (2010) Hydrated metal ions in aqueous solution: how regular are their structures? Pure Appl Chem 82:1901–1917. https://doi.org/10.1351/PAC-CON-09-10-22
Wander MCF, Clark AE (2008) Hydration properties of aqueous Pb(II) ion. Inorg Chem 46:8233–8241. https://doi.org/10.1021/ic800750g
Kelly CP, Cramer CJ, Truhlar DG (2005) SM6: a density functional theory continuum solvation model for calculating aqueous solvation free energies of neutrals, ions, and solute-water clusters. J Chem Theory Comput 1:1133–1152. https://doi.org/10.1021/ct050164b
Kelly CP, Cramer CJ, Truhlar DG (2006) Aqueous solvation free energies of ions and ion-water clusters based on an accurate value for the absolute aqueous solvation free energy of the proton. J Phys Chem B 110:16066–16081. https://doi.org/10.1021/jp063552y
Eisenberg D, Kauzmann W (1969) The structure and properties of water. Oxford University Press, London
Shimoni-Livny L, Glusker JP, Bock CW (1998) Lone pair functionality in divalent lead compounds. Inorg Chem 37:1853–1867. https://doi.org/10.1021/ic970909r
Swift TJ, Sayre WG (1966) Determination of hydration numbers of cations in aqueous solution by means of proton NMR. J Chem Phys 44:3567–3574. https://doi.org/10.1063/1.1727266
Bargar JR, Brown GEJ, Parks GA (1997) Surface complexation of Pb(II) at oxide-water interfaces: I. XAFS and bond-valence determination of mononuclear and polynuclear Pb(II) sorption products on aluminum oxides. Geochim Cosmochim Acta 61:2617–2637. https://doi.org/10.1016/S0016-7037(97)00124-5
Persson I, Lyczko K, Lundberg D, Eriksson L, Płaczek A (2011) Coordination chemistry study of hydrated and solvated lead(II) ions in solution and solid state. Inorg Chem 50:1058–1072. https://doi.org/10.1021/ic1017714
Gourlaonen C, Gerard H, Parisel O (2006) Exploring the hydration of Pb2+: Ab initio studies and first principles molecular dynamics. Chem Eur J 12:5024–5032. https://doi.org/10.1002/chem.200600045
Bhattcharjee A, Hofer TS, Pribil AB, Randolf BR, Lim LHV, Lichtenberger AF, Rode BM (2009) Revisiting the hydration of Pb(II): a QMCF MD approach. J Phys Chem B 113:13007–13013. https://doi.org/10.1021/jp905848x
Marcus Y (1987) The thermodynamics of solvation of ions. Part 2. The enthalpy of hydration at 298.15 K. J Chem Soc Faraday Trans 83:339–349. https://doi.org/10.1039/F19878300339
Marcus Y (1991) Thermodynamics of solvation of ions. Part 5. Gibbs free energy of hydration at 298.15 K. J Chem Soc Faraday Trans 87:2995–2999. https://doi.org/10.1039/FT9918702995
Canal Neto A, Jorge FE (2013) All-electron double zeta basis sets for the most fifth-row atoms: application in DFT spectroscopic constant calculations. Chem Phys Lett 582:158–162
Liptak MD, Shields GC (2001a) Experimentation with different thermodynamic cycles used for pK a calculations on carboxylic acids using complete basis set and Gaussian-n models combined with CPCM continuum solvation methods. Int J Quantum Chem 85:727–741. https://doi.org/10.1002/qua.1703
Tawa GJ, Topol IA, Burt SK, Caldwell RA, Rashin AA (1998) Calculation of the aqueous solvation free energy of the proton. J Chem Phys 109:4852–4863. https://doi.org/10.1063/1.477096
Liptak MD, Shields GC (2001b) Accurate pK a calculations for carboxylic acids using complete basis set and Gaussian-n models combined with CPCM continuum solvation methods. J Amer Chem Soc 123:7314–7319. https://doi.org/10.1021/ja010534f
Liptak MD, Gross KC, Saybold PG, Feldgus S, Shields GC (2002) Absolute pK a determinations for substituted phenols. J Amer Chem Soc 124:6421–6427. https://doi.org/10.1021/ja012474j
Benjamin MM (2015) Water chemistry, 2nd edn, Chap. 10. Waveland, Long Grove, pp 561–566
Pankow JF (1991) Aquatic chemistry concepts, Chap. 11. Lewis, Chelsea, pp 217–242
Weng CH (2004) Modeling Pb(II) adsorption onto sandy loam soil. J Colloid Interface Sci 272:262–270. https://doi.org/10.1016/j.jcis.2003.11.051
Baes CF Jr, Mesmer RE (1976) The hydrolysis of cations, Chap. 15. Wiley, New York, pp 358–365 (and references therein)
Cruywagen JJ, van de Water RF (1993) The hydrolysis of lead(II). A potentiometric and enthalpimetric study. Talanta 40:1091–1095. https://doi.org/10.1016/0039-9140(93)80171-M
Seybold PG, Shields GC (2015) Computational estimation of pK a values. WIREs Comput Mol Sci 5:290–297. https://doi.org/10.1002/wcms.1218
Li J, Fisher CL, Chen JL, Bashford D, Noodleman L (1996) Calculation of redox potentials and pK a values of hydrated transition metal cations by a combined density functional and continuum dielectric theory. Inorg Chem 35(16):694–4702. https://doi.org/10.1021/ic951428f
De Abreu HA, Guimarães L, Duarte HA (2006) Density-functional theory study of iron(III) hydrolysis in aqueous solution. J Phys Chem A 110(24):7713–7718. https://doi.org/10.1021/jp060714h
Gilson R, Durrant MC (2009) Estimation of the pK a values of water ligands in transition metal complexes using density functional theory with polarized continuum model solvent corrections. Dalton Trans 46:10223–10230 https://doi.org/10.1039/C7DT04291D
Yang W, Qian Z, Miao Q, Wang Y, Bi S (2009) Density functional theory study of the aluminium(III) hydrolysis in aqueous solution. Phys Chem Chem Phys 11:2396–2401. https://doi.org/10.1039/B819484J
Jackson VE, Felmy AR, Dixon DA (2015) Prediction of the pK a’s of aqueous metal ion +2 complexes. J Phys Chem A 119:2926–2939. https://doi.org/10.1021/jp5118272
Galstyan G, Knapp EW (2015) Computing pK a values of hexa-aqua transition metal complexes. J Comput Chem 36:69–78. https://doi.org/10.1002/jcc.23764
Yu D, Du R, Xiao J-C, Xu S, Rong C, Liu S (2018) Theoretical study of pK a values for trivalent rare-earth metal cations in aqueous solution. J Phys Chem A 122(2):700–707. https://doi.org/10.1021/acs.jpca.7b12074
Acknowledgments
This work was supported by the Ministry of Education and Science of the Russian Federation (Project No.5382.2017).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
Supplementary data associated with this article (four tables presenting Cartesian coordinates of Pb(II) complexes modeled in this study, hydration energies of Pb(II) ion and the averaged Pb-O distances in hexa-aqua complex calculated using the all-electron basis set for Pb, Gibbs free energy and pKa1 value for the hydrolysis of Pb(II) ion determined for different initial composition of the aqua complexes) can be found in the online version of this paper. (DOCX 27 kb)
Rights and permissions
About this article
Cite this article
Kuznetsov, A.M., Masliy, A.N. & Korshin, G.V. Quantum-chemical simulations of the hydration of Pb(II) ion: structure, hydration energies, and pKa1 value. J Mol Model 24, 193 (2018). https://doi.org/10.1007/s00894-018-3726-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-018-3726-4