Abstract
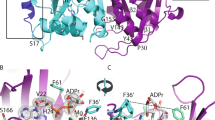
Peptidoglycan (PGN) recognition proteins (PGRPs) are important pattern recognition receptors of the innate immune system. A number of PGRP splicing variants produced by alternative splicing of PGRP genes have been reported. However, several important aspects of interactions between PGRP splice variants and their ligands are still unclear. In the present study, three dimensional models of salamander PGRP1 (adPGRP1) and its splice variant (adPGRP1a) were constructed, and their key amino acids involved in interacting with PGNs were analyzed. The results revealed that adPGRP1a has a typical PGRPs structure containing five β-sheets and four α-helices, while adPGRP1 contained five β-sheets and only one α-helix due to the lack of 51 amino acids at its C-terminus. Molecular docking revealed that van der Waals and Coulombic interactions contributed to interactions in the protein–ligand complex. Further binding energy of adPGRP-PGNs computed by the MM-PBSA method revealed that adPGRP1a and adPGRP1 might selectively bind to different PGNs; the former might selectively bind Dap-type PGNs and the latter both types of PGNs. In addition, the binding energy of each residue of adPGRP1a and adPGRP1 was also calculated, revealing that residues involved in the interaction of protein–ligand complexes were different in adPGRP1a and adPGRP1. These results provided a first insight into the potential basis for interaction between PGRPs generated by alternative splicing and PGN derivatives.









Similar content being viewed by others
References
Barbé F, Douglas T, Saleh M (2014) Advances in Nod-like receptors (NLR) biology. Cytokine Growth Factor Rev 25:681–697. doi:10.1016/j.cytogfr.2014.07.001
Medzhitov R, Janeway JC (2000) Innate immune recognition: mechanisms and pathways. Immunol Rev 173:89–97. doi:10.1034/j.1600-065X.2000.917309.x
Janeway JC, Medzhitov R (2002) Innate immune recognition. Annu Rev Immunol 20:197–216. doi:10.1146/annurev.immunol.20.083001.084359
Rosenfeld Y, Shai Y (2006) Lipopolysaccharide (endotoxin)-host defense antibacterial peptides interactions: role in bacterial resistance and prevention of sepsis. Biochim Biophys Acta 1758:1513–1522. doi:10.1016/j.bbamem.2006.05.017
Dziarski R (2004) Peptidoglycan recognition proteins (PGRPs). Mol Immunol 40:877–886. doi:10.1016/j.molimm.2003.10.011
Yoshida H, Kinoshita K, Ashida M (1996) Purification of a peptidoglycan recognition protein from hemolymph of the silkworm, Bombyx mori. J Biol Chem 271:13854–13860
Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D (2000) A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA 97:13772–13777. doi:10.1073/pnas.97.25.13772
Su J, Ni D, Song L, Zhao L, Qiu I (2007) Molecular cloning and characterization of a short peptidoglycan recognition protein (CfPGRP-S1) cDNA from Zhikong scallop Chlamys farreri. Fish Shellfish Immunol 23:646–656. doi:10.1016/j.fsi.2007.01.023
Coteur G, Mellroth P, De Lefortery C, Gillan D, Dubois P, Communi D (2007) Peptidoglycan recognition proteins with amidase activity in early deuterostomes (Echinodermata). Dev Comp Immunol 31:790–804. doi:10.1016/j.dci.2006.11.006
Chang MX, Nie P, Wei LL (2007) Short and long peptidoglycan recognition proteins (PGRPs) in zebrafish, with finding of multiple PGRP homologs in teleost fish. Mol Immunol 44:3005–3023. doi:10.1016/j.molimm.2006.12.029
Qi ZT, Zhang QH, Wang ZS, Wang AM, Huang B, Chang MX, Nie P (2011) Cloning and expression of a long type peptidoglycan recognition protein (PGRP-L) from Xenopus tropicalis. Zool Res 32:371–378. doi:10.3724/SP.J.1141.2011.04371
Liu C, Xu Z, Gupta D, Dziarski R (2001) Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem 276:34686–34694
Garver LS, Wu J, Wu LP (2006) The peptidoglycan recognition protein PGRP-SCa is essential for Toll signaling and phagocytosis of Staphylococcus aureus in Drosophila. Proc Natl Acad Sci USA 103:660–665. doi:10.1073/pnas.0506182103
Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, Oshima Y, Peach C, Erturk-Hasdemir D, Goldman WE, Oh BH, Kurata S, Silverman N (2006) PGRP-LC and PGRP-LE have essential yet distinct function in the Drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol 7:715–723. doi:10.1038/ni1356
Paredes JC, Welchman DP, Poidevin M, Lemaitre B (2011) Negative regulation by amidase PGRPs shapes the Drosophila antibacterial responses and protects the fly from innocuous infection. Immunity 35:770–779. doi:10.1016/j.immuni.2011.09.018
Li X, Wang S, Qi J, Echtenkamp SF, Chatterjee R, Wang M, Boons GJ, Dziarski R, Gupta D (2007) Zebrafish peptidoglycan recognition proteins are bactericidal amidases for defense against bacterial infections. Immunity 27:518–529. doi:10.1016/j.immuni.2007.07.020
Zhang Y, van der Fits L, Voerman JS, Melief MJ, Laman JD, Wang M, Wang H, Wang M, Li X, Walls CD, Gupta D, Dziarski R (2005) Identification of serum N-acetylmuramoyl-l-alanine amidase as liver peptidoglycan recognition protein 2. Biochim Biophys Acta 1752:34–46. doi:10.1016/j.bbapap.2005.07.001
Michel T, Reichhart JM, Hoffmann JA, Royet J (2001) Drosophila Toll is activated by gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 13:756–759. doi:10.1038/414756a
Leone P, Bischoff V, Kellenberger C, Hetru C, Royer J, Roussel A (2008) Crystal structure of Drosophila PGRP-SD suggests binding to Dap-type but not lysine-type peptidoglycan. Mol Immunol 45:2521–2530. doi:10.1016/j.molimm.2008.01.015
Cho JH, Fraser IP, Fukase K, Kusumoto S, Fujimoto Y, Stahl GL, Ezekowitz AB (2005) Human peptidoglycan recognition protein S is an effector of neutrophil-mediated innate immunity. Blood 106:2551–2558. doi:10.1182/blood-2005-02-0530
Tydell CC, Yount N, Tran D, Yuan J, Selsted ME (2002) Isolation, characterization, and antimicrobial properties of bovine oligosaccharide-binding protein. J Biol Chem 277:19658–19664
Meister S, Agianian B, Turlure F, Relogio A, Morlais I, Kafatos FC, Christophides GK (2009) Anopheles gambiae PGRP-LC mediated defense against bacterial modulates infections with malaria parasites. PLoS Pathog 5:e1000542. doi:10.1371/journal.ppat.1000542
Yu ZL, Li JH, Xue NN, Nie P, Chang MX (2014) Expression and functional characterization of PGRP6 splice variants in grass carp, Ctenopharyngodon idella. Dev Comp Immunol 47:264–274. doi:10.1016/j.dci.2014.08.006
Kibardin AV, Mirkina LL, Baranova EV, Zakeyeva LR, Georgiev GP, Kiselev SL (2003) The differentially spliced mouse tagL gene, homolog of tag7/PGRP gene family in mammals and Drosophila, can recognize gram-positive and gram-negative bacterial cell wall independently of T phage lysozyme homology domain. J Mol Biol 326:467–474. doi:10.1016/S0022-2836(02)01401-8
Kaneko T, Goldman WE, Mellroth P, Steriner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N (2004) Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity 20:637–649. doi:10.1016/S1074-7613(04)00104-9
Qi ZT, Zhang QH, Wang ZS, Ma TY, Zhou J, Holland JW, Gao Q (2016) Transcriptome analysis of the endangered Chinese giant salamander (Andrias davidianus): immune modulation in response to Aeromonas hydrophila infection. Vet Immunol Immunop 169:85–95. doi:10.1016/j.vetimm.2015.11.004
Petersen TN, Brunak S, von Heijne G, Nielsen H (2011) SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi:10.1038/nmeth.1701
Krogh A, Larsson B, von Heijne G, Sonnhammer EL (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 305:567–580. doi:10.1006/jmbi.2000.4315
Wilkins MR, Gasteiger E, Bairoch A, Sanchez JC, Williams KL, Appel RD, Hochstrasser DF (1999) Protein Identification and Analysis Tools in the ExPASy Server. Methods Mol Biol 112:531–552
Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, Boursnell C, Pang N, Forslund K, Ceric G, Clements J, Heger A, Holm L, Sonnhammer ELL, Eddy SR, Bateman A, Finn RD (2012) The pfam protein families database. Nucleic Acids Res 40:D290–D301. doi:10.1093/nar/gkr1065
Levine A, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40:D302–D305. doi:10.1093/nar/gkr931
Marchler-Bauer A, Lu S, Anderson JB, Chitsaz F, Derbyshire MK, DeWeese-Scott C et al (2011) CDD: a conserved domain database for the functional annotation of proteins. Nucleic Acids Res 39:D225–229. doi:10.1093/nar/gkq1189
Zhang Y (2008) I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9:40. doi:10.1186/1471-2105-9-40
Kurowski MA, Bujnicki JM (2003) GeneSilico protein structure prediction meta-server. Nucleic Acids Res 31:3305–3307. doi:10.1093/nar/gkg557
Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK-a program to check the stereochemical quality of protein structures. J App Cryst 26:283–291. doi:10.1107/S0021889892009944
Colovos C, Yeates TO (1993) Verification of protein structures: patterns of non-bonded atomic interactions. Protein Sci 2:1511–1519. doi:10.1002/pro.5560020916
Lüthy R, Bowie JU, Eisenberg D (1992) Assessment of protein models with three-dimensional profiles. Nature 356:83–85. doi:10.1038/356083a0
Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallographica D 66:12–21. doi:10.1107/S0907444909042073
Wiederstein M, Sippl MJ (2007) ProSA-web, interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acid Res 35:407–410. doi:10.1093/nar/gkm290
Wallner B, Elofsson A (2003) Can correct protein models be identified? Protein Sci 12:1073–1086. doi:10.1110/ps.0236803
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65:712–725. doi:10.1002/prot.21123
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through muti-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. doi:10.1016/j.softx.2015.06.001
Kumari R, Kumar R, Consortium OSDD, Lynn A (2014) g_mmpbsa--a GROMACS tool for high-throughput MM-PBSA calculations. J Chem Inf Model 54:1951–1962. doi:10.1021/ci500020m
Sahoo BR, Dubey PK, Goyal S, Bhoi GK, Lenka SK, Maharana J, Pardhan SK, Kataria RS (2014) Exploration of the binding modes of buffalo PGRP1 receptor complexed with meso-diaminopimelic acid and lysine-type peptidoglycans by molecular dynamics simulation and free energy calculation. Chem Biol Interact 220:255–268. doi:10.1016/j.cbi.2014.06.028
Guan R, Wang Q, Sundberg EJ, Mariuzza RA (2005) Crystal structure of human peptidoglycan recognition protein S (PGRP-S) at 1.70 A resolution. J Mol Biol 347:683–691. doi:10.1016/j.jmb.2005.01.070
Guan R, Malchiodi EL, Qian W, Schuck P, Mariuzza RA (2004) Crystal structure of the C-terminal peptidoglycan-binding domain of human peptidoglycan recognition protein Ialpha. J Biol Chem 279:31873–31882. doi:10.1074/jbc.M404920200
Genheden S, Rede U (2015) The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin Drug Discov 10:449–461. doi:10.1517/17460441.2015.1032936
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 31302221, 31272666 and 31470130) and Jiangsu Province (Grant no. BK2011418 and BK20151297), and partially by the Major Projects of Natural Science Research in Universities and Colleges in Jiangsu Province (Grant no. 15KJA240001). Z.Q. was financially supported by the “Qinglan” project of Jiangsu province of China.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Qi, Z., Meng, F., Zhang, Q. et al. Structural insights into ligand binding of PGRP1 splice variants in Chinese giant salamander (Andrias davidianus) from molecular dynamics and free energy calculations. J Mol Model 23, 135 (2017). https://doi.org/10.1007/s00894-017-3315-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3315-y