Abstract
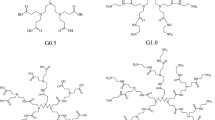
In this work, the molecular modeling method was performed to study adsorption interaction between PAMAM molecules of different generations and silicic acid molecules, and the inhibition effect on silica scale were discussed. The results show that adsorption energies of PAMAM molecule of generation 1.0 with amine-terminated groups are stronger than those of generation 1.5 with terminated carboxyl group. The composition of adsorption interactions are the dominating electrostatic interactions and van de Waals interactions as well as H-bond interactions. It is qualitatively discussed that the inhibition effect of generation 1.0 on silica scale is stronger than that of generation 1.5 in the neutral solution.




Similar content being viewed by others
References
Mora‐Fonz MJ, Catlow CRA, Lewis DW (2005) Oligomerization and cyclization processes in the nucleation of microporous silicas. Angewandte Chemie International Edition 44 (20):3082-3086
Bremere I, Kennedy M, Mhyio S, Jaljuli A, Witkamp G-J, Schippers J (2000) Prevention of silica scale in membrane systems: removal of monomer and polymer silica. Desalination 132(1):89–100
Tomalia D, Baker H, Dewald J, Hall M, Kallos G, Martin S, Roeck J, Ryder J, Smith P (1985) A new class of polymers: starburst-dendritic. Polym J 17(1):117–132
Tomalia DA (2005) Birth of a new macromolecular architecture: dendrimers as quantized building blocks for nanoscale synthetic polymer chemistry. Prog Polym Sci 30(3):294–324
Esfand R, Tomalia DA (2001) Poly (amidoamine)(PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today 6(8):427–436
Yiyun C, Jiepin Y (2005) Effect of polyamidoamine dendrimers in decolorising triarylmethane dye effluent. Color Technol 121(2):72–75
Diallo MS, Christie S, Swaminathan P, Johnson JH, Goddard WA (2005) Dendrimer enhanced ultrafiltration. 1. Recovery of Cu (II) from aqueous solutions using PAMAM dendrimers with ethylene diamine core and terminal NH2 groups. Environ Sci Technol 39(5):1366–1377
Zhang B, Sun P, Chen F, Li F (2012) Synergistic inhibition effect of polyaminoamide dendrimers and polyepoxysuccinic acid on silica polymerization. Colloids Surf A Physicochem Eng Asp 410:159–169
Demadis KD (2005) A structure/function study of polyaminoamide dendrimers as silica scale growth inhibitors. J Chem Technol Biotechnol 80(6):630–640
Mavredaki E, Neofotistou E, Demadis KD (2005) Inhibition and dissolution as dual mitigation approaches for colloidal silica fouling and deposition in process water systems: functional synergies. Ind Eng Chem Res 44(17):7019–7026
Neofotistou E, Demadis KD (2004) Use of antiscalants for mitigation of silica (SiO 2) fouling and deposition: fundamentals and applications in desalination systems. Desalination 167:257–272
Hädicke E, Rieger J, Rau IU, Boeckh D (1999) Molecular dynamics simulations of the incrustation inhibition by polymeric additives. Phys Chem Chem Phys 1(17):3891–3898
Chen C, Lei W, Xia M, Wang F, Gong X (2013) Molecular modeling of several phosphonates onto the stepped calcite (011) surface. Desalination 309:208–212
Chen C, Xia M, Wu L, Zhou C, Wang F (2012) Modeling the interaction of seven bisphosphonates with the hydroxyapatite (100) face. J Mol Model 18(9):4007–4012
Shi W, Xia M, Lei W, Wang F (2013) Molecular dynamics study of polyether polyamino methylene phosphonates as an inhibitor of anhydrite crystal. Desalination 322:137–143
Shi W-Y, Ding C, Yan J-L, Han X-Y, Lv Z-M, Lei W, Xia M-Z, Wang F-Y (2012) Molecular dynamics simulation for interaction of PESA and acrylic copolymers with calcite crystal surfaces. Desalination 291:8–14
Zhang S, Lei W, Xia M, Wang F (2005) QSAR study on N-containing corrosion inhibitors: quantum chemical approach assisted by topological index. J Mol Struct THEOCHEM 732(1):173–182
Discover/Accelrys (2007) Materials studio 4.0. Discover/Accelrys, San Diego
Sheikholeslami R, Tan S (1999) Effects of water quality on silica fouling of desalination plants. Desalination 126(1):267–280
Sun H (1998) COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds. J Phys Chem B 102(38):7338–7364
Chen G, Xia M, Lei W, Wang F, Gong X (2014) Prediction of crystal morphology of cyclotrimethylene trinitramine in the solvent medium by computer simulation: a case of cyclohexanone solvent. J Phys Chem A 118(49):11471–11478
Chen G, Xia M, Lei W, Wang F, Gong X (2013) A study of the solvent effect on the morphology of RDX crystal by molecular modeling method. J Mol Model 19(12):5397–5406
Chen G, Xia M, Lei W, Wang F, Gong X (2014) Molecular dynamics investigation of the effect of solvent adsorption on crystal habits of hexogen. Can J Chem 92(9):849–854
Zhong T, Ai P, Zhou J (2011) Structures and properties of PAMAM dendrimer: A multi-scale simulation study. Fluid Phase Equilib 302(1):43–47
Martinho N, Florindo H, Silva L, Brocchini S, Zloh M, Barata T (2014) Molecular modeling to study dendrimers for biomedical applications. Molecules 19(12):20424–20467
Desiraju GR (2002) Hydrogen bridges in crystal engineering: interactions without borders. Acc Chem Res 35(7):565–573
Li C, Choi P (2008) Molecular dynamics study on the effect of solvent adsorption on the morphology of glycothermally produced α-Al2O3 particles
Vail JG (1952) Soluble silicates. Soil Sci 74(5):407
Chan S, Chen Z, He P (1995) Effect of ferric chloride on silica fouling. J Heat Transf 117(2):323–328
Belton DJ, Deschaume O, Perry CC (2012) An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances. FEBS J 279(10):1710–1720
Lee I, Athey BD, Wetzel AW, Meixner W, Baker JR (2002) Structural molecular dynamics studies on polyamidoamine dendrimers for a therapeutic application: effects of pH and generation. Macromolecules 35(11):4510–4520
Demadis KD, Neofotistou E (2007) Synergistic effects of combinations of cationic polyaminoamide dendrimers/anionic polyelectrolytes on amorphous silica formation: a bioinspired approach. Chem Mater 19(3):581–587
Acknowledgements
This work was funded by “Jiangsu Youth Funding Program SBK2015041911”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, C., Bai, N., Zhang, Y. et al. A theoretical study of the inhibition effect of PAMAM molecule on silica scale. J Mol Model 23, 32 (2017). https://doi.org/10.1007/s00894-017-3208-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-017-3208-0