Abstract
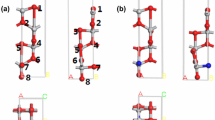
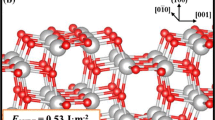
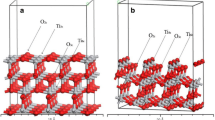
Lithiation of TiO2 has been shown to enhance the storage of hydrogen up to 5.6 wt% (Hu et al. J Am Chem Soc 128:11740–11741, 2006). The mechanism for the process is still unknown. In this work we have carried out a study on the adsorption and diffusion of Li atoms on the surface and migration into subsurface layers of anatase (101) by periodic density functional theory calculations implementing on-site Coulomb interactions (DFT+U). The model consists of 24 [TiO2] units with 11.097 × 7.655 Å2 surface area. Adsorption energies have been calculated for different Li atoms (1–14) on the surface. A maximum of 13 Li atoms can be accommodated on the surface at two bridged O, Ti-O, and Ti atom adsorption sites, with 83 kcal mol−1 adsorption energy for a single Li atom adsorbed between two bridged O atoms from where it can migrate into the subsurface layer with 27 kcal mol−1 energy barrier. The predicted adsorption energies for H2 on the lithiated TiO2 (101) surface with 1–10 Li atoms revealed that the highest adsorption energies occurred on 1-Li, 5-Li, and 9-Li surfaces with 3.5, 4.4, and 7.6 kcal mol−1, respectively. The values decrease rapidly with additional H2 co-adsorbed on the lithiated surfaces; the maximum H2 adsorption on the 9Li-TiO2(a) surface was estimated to be only 0.32 wt% under 100 atm H2 pressure at 77 K. The result of Bader charge analysis indicated that the reduction of Ti occurred depending on the Li atoms covered on the TiO2 surface.






Similar content being viewed by others
References
Fujishima A, Honda K (1972) Nature 238:37–38
Chen X, Shen S, Guo L, Mao SS (2010) Chem Rev 110:6503–6570
Chen X, Liu L, Yu PY, Mao SS (2011) Science 331:746–750
Sun C, Jia Y, Yang XH, Yang HG, Yao X, Lu GQ, Selloni A, Smith SC (2011) J Phys Chem C 115:25590–25594
Zheng Z, Huang B, Lu J, Wang Z, Qin X, Zhang X, Dai Y, Whangbo MH (2012) Chem Commun 48:5733–5735
Tarnawski Z, Kim-Ngan NH, Zakrzewska K, Drogowska K, Brudnik A, Balogh AG, Kuzel R, Havela L, Sechovsky V (2013) Adv Nat Sci Nanosci Nanotechnol 4:025004
Mao SS, Shen S, Guo L (2012) Prog Nat Sci Mater Int 22:522–534
Hu X, Skadtchenko BO, Trudeau M, Antonelli DM (2006) J Am Chem Soc 128:11740–11741
Bavykin DV, Lapkin AA, Plucinski PK, Friedrich JM, Walsh FC (2005) J Phys Chem B 109:19422–19427
Lim SH, Luo J, Zhong Z, Ji W, Lin J (2005) Inorg Chem 44:4124–4126
Wang G, Wang H, Ling Y, Tang Y, Yang X, Fitzmorris RC, Wang C, Zhang JZ, Li Y (2011) Nano Lett 11:3026–3033
Raghunath P, Huang WF, Lin MC (2013) J Chem Phys 138:154705
Kresse G, Furthmüller J (1996) Phys Rev B 54:11169–11186
Kresse G, Hafner J (1993) Phys Rev B 47:558–561
Blöchl PE (1994) Phys Rev B 50:17953–17979
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Huang WF, Raghunath P, Lin MC (2011) J Comput Chem 32:1065–1081
Raghunath P, Lin MC (2008) J Phys Chem C 112:8276–8287
Monkhorst HJ, Pack JD (1967) Phys Rev B 13:5188–5192
Henkelman G, Uberuaga BP, Jónsson H (2000) J Chem Phys 113:9901–9904
Hüfner S (1994) Adv Phys 43:183–356
Finazzi E, Valentin CD, Pacchioni G, Selloni A (2008) J Chem Phys 129:154113
Wagemaker M, Krol RVD, Kentgens APM, van Well AA, Mulder FM (2001) J Am Chem Soc 123:11454–11461
Lunell S, Stashans A, Ojamae L, Lindstrom H, Hagfeldt A (1997) J Am Chem Soc 119:7374–7380
Lin F, Zhou G, Li Z, Li J, Wu J, Duan W (2009) Chem Phys Lett 475:82–85
Thompson TL, Yates JT (2006) Chem Rev 106:4428–4453
Birowska M, Milowska K, Majewski JA (2011) Acta Phys Pol A 120:845–848
Dobson JF, Gould T (2012) J Phys Condens Matter 24:073201
Chen DL, Al-Saidi WA, Johnson JK (2012) J Phys Condens Matter 24:424211
Forrer D, Vittadini A (2011) Chem Phys Lett 516:72–75
Moellmann J, Ehrlich S, Tonner R, Grimme S (2012) J Phys Condens Matter 24:424206
Henkelman G, Arnaldsson A, Jonsson H (2006) Comput Mater Sci 36:354–360
Serpone N (2006) J Phys Chem B 110:24287–24293
Fujishima A, Zhang X, Tryk DA (2008) Surf Sci Rep 63:515–582
Gratzel MJ (2003) Photochem Photobiol C 4:145–153
Mokrushin V, Bedanov V, Tsang W, Zachariah M, Knyazev V (2002) ChemRate Version 1.19. National Institute of Standards and Technology, Gaithersburg
Acknowledgments
This research was supported by the National Science Council (NSC) of Taiwan. We acknowledge the National Center for High-performance Computing for providing the computer time. M.C.L. thanks the National Science Council of Taiwan for the distinguished visiting professorship at NCTU.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supporting information
The PES of Li migration from surface to subsurface is shown in Fig. S1. PES of 2Li migration and its geometries are shown in Figs. S2-S3. Adsorption energies of 1H2 on (1–10)Li-TiO2(a) and (1–5)H2 on (1,5,9)Li-TiO2(a) are shown in Tables S1-13. Their related geometries are shown in Figs. S4-S7. H2 adsorption on Li-TiO2(001) surface is shown in Fig. S8. (DOCX 20704 kb)
Rights and permissions
About this article
Cite this article
Srinivasadesikan, V., Raghunath, P. & Lin, M.C. Quantum chemical investigation on the role of Li adsorbed on anatase (101) surface nano-materials on the storage of molecular hydrogen. J Mol Model 21, 142 (2015). https://doi.org/10.1007/s00894-015-2686-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2686-1