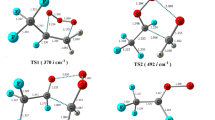
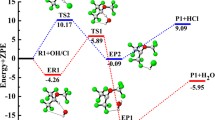
Abstract
In the present work, theoretical study on the mechanism and kinetics of the gas-phase reactions of CF3CF2CH2OCH3 (HFE-365mcf3) with the OH radicals have been performed using meta-hybrid modern density functional M06-2X in conjunction with 6-31+G(d,p) basis set. Reaction profiles for OH-initiated hydrogen abstraction are modeled including the formation of pre-reactive and post-reactive complexes at entrance and exit channels. Our calculations reveal that hydrogen abstraction from the –CH2 group is thermodynamically more facile than that from the –CH3 group. This is further ascertained by the calculated C-H bond dissociation energy of CF3CF2CH2OCH3 molecule. The rate constants of the titled reactions are computed over the temperature range of 250–450 K. The calculated rate constant value at 298 K is found to be in reasonable agreement with the experimental results. The atmospheric life time of HFE-365mcf3 is estimated to be 42 days. The atmospheric fate of the alkoxy radicals, CF3CF2CH(O•)OCH3 and CF3CF2CH2OCH2O• are also investigated for the first time using the same level of theory. Out of three plausible decomposition channels, our results clearly point out that reaction with O2 is the dominant atmospheric sink for the decomposition of CF3CF2CH(O•)OCH3 radical in the atmosphere.


Similar content being viewed by others
References
Sekiya A, Misaki S (2000) J Fluor Chem 101:215–221
Powell RL (2002) J Fluor Chem 114:237–250
McCulloch A (1999) J Fluor Chem 100:163–173
Imasu R, Suga A, Matsuno T (1995) J Meteorol Soc Jpn 73:1123–1136
Blowers P, Tetrault KF, Morehead YT (2008) Theor Chem Accounts 119:369–381
Blanco MB, Bejan I, Barnes I, Wiesen P, Teruel MA (2010) Environ Sci Technol 44(7):2354–2359
Bravo I, Dıaz-de-Mera Y, Aranda A, Moreno E, Nutt DR, Marston G (2011) Phys Chem Chem Phys 13:17185–17193
Ninomiya Y, Kawasaki M, Guschin A, Molina LT, Molina MJ, Wallington TJ (2000) Environ Sci Technol 34(14):2973–2978
Oyaro N, Sellevåg SR, Nielsen CJ (2004) Environ Sci Technol 38:5567–5576
Thomsen DL, Andersen VF, Nielsen OJ, Wallington TJ (2011) Phys Chem Chem Phys 13:2758–2764
Urata S, Takada A, Uchimaru T, Chandra AK (2003) Chem Phys Lett 368:215–223
Mishra BK, Lily M, Chakrabartty AK, Bhattacharjee D, Deka RC, Chandra AK (2014) New J Chem 38:2813–2822
Mishra BK (2014) J Mol Model 20:2444
Mishra BK, Deka RC (2014) J Phys Chem A 118(38):8779–8786
Singh HJ, Mishra BK, Rao PK (2012) Can J Chem 90(4):403–409
Singh HJ, Gour NK, Srivastava P (2013) Mol Phys 111:3756–3761
Singh HJ, Mishra BK, Gour NK (2010) Theor Chem Accounts 125:57–64
Mishra BK (2014) RSC Adv 4(32):16759–16764
Dalmasso PR, Taccone RA, Nieto JD, Cometto PM, Cobos CJ, Lane SI (2014) Atmos Environ 91:104–109
Chandra AK, Uchimaru T (2001) Chem Phys Lett 334:200–206
Zhao Y, Truhlar DG (2008) Theor Chem Accounts 120:215–241
Beste A, Buchanan AC III (2010) Energy Fuels 24:2857–2867
Dinadayalane TC, Paytakov G, Leszczynski J (2013) J Mol Model 19:2855–2864
Lily M, Mishra BK, Chandra AK (2014) J Fluor Chem 161:51–59
Mishra BK, Lily M, Chandra AK, Deka RC (2014) J Phys Org Chem 27(10):811–817
Sandhiya L, Kolandaivel P, Senthilkumar K (2012) Struct Chem 23:1475–1488
Mandal D, Sahu C, Bagchi S, Das AK (2013) J Phys Chem A 117:3739–3750
Chandra AK (2012) J Mol Model 18:4239–4247
Devi Kh J, Chandra AK (2011) Chem Phys Lett 502:23–28
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Frisch MJ et al. (2009) GAUSSIAN 09 (Revision B.01). Gaussian Inc, Wallingford
Hammond GS (1955) J Am Chem Soc 77:334–338
Beste A, Buchanan AC III (2012) Chem Phys Lett 550:19–24
Younker JM, Beste A, Buchanan AC III (2012) Chem Phys Lett 545:100–106
Csontos J, Rolik Z, Das S, Kallay M (2010) J Phys Chem A 114:13093–13103
Kondo S, Takahashi A, Tokuhashi K, Sekiya A, Yamada Y, Saito K (2002) J Fluor Chem 117:47–53
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, New Delhi
Brown RL (1981) J Res Natl Bur Stand 86:357–359
Xiao R, Noerpel M, Luk HL, Wei Z, Spinney R (2014) Int J Quantum Chem 114:74–83
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221–1228
Singleton DL, Cvetonovoic RJ (1976) J Am Chem Soc 98:6812–6819
Blanco MB, Rivela C, Teruel MA (2013) Chem Phys Lett 578:33–37
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastridge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Nature 394:353–355
Spivakovsky CM, Logan JA, Montzka SA, Balkanski YJ, Foreman-Fowler M, Jones DBA, Horowitz LW, Fusco AC, Brenninkmeijer CAM, Prather MJ, Wofsy SC, McElroy MB (2000) J Geophys Res 105(D7):8931–8980
Bravo I, Aranda A, Hurley MD, Marston G, Nutt DR, Shine KP, Smith K, Wallington TJ (2010) J Geophys Res 115, D24317
Mishra BK, Lily M, Deka RC, Chandra AK (2014) J Mol Graphics Model 50:90–99
Acknowledgments
DB acknowledges CSIR, New Delhi, for financial assistance in form of Senior Research Fellowship. BKM is thankful to University Grants Commission, New Delhi for providing Dr. D. S. Kothari Fellowship.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 722 kb)
Rights and permissions
About this article
Cite this article
Bhattacharjee, D., Mishra, B.K. & Deka, R.C. Theoretical insight on atmospheric chemistry of HFE-365mcf3: reactions with OH radicals, atmospheric lifetime, and fate of alkoxy radicals (CF3CF2CH(O•)OCH3/CF3CF2CH2OCH2O•). J Mol Model 21, 69 (2015). https://doi.org/10.1007/s00894-015-2629-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-015-2629-x