Abstract
The mechanism and kinetics of the radical 3C2 + C3H8 reaction have been investigated theoretically by direct ab initio kinetics over a wide temperature range. The potential energy surfaces have been constructed at the CCSD(T)/B3//UMP2/B1 levels of theory. The electron transfer was also analyzed by quasi–restricted orbital (QRO) in detail. It was shown that all these channels proceed exclusively via hydrogen abstraction. The overall ICVT/SCT rate constants are in agreement with the available experimental results. The prediction shows that the secondary hydrogen of C3H8 abstraction by 3C2 radical is the major pathway at low temperatures (below 700 K), while as the temperature increases, the primary hydrogen of C3H8 abstraction becomes more important and more favorable. A negative temperature dependence of the rate constants for the reaction of 3C2 + C3H8 was observed. The three–(k 3) and four–parameter (k 4) rate-temperature expressions were also provided within 243–2000 K to facilitate future experimental studies.

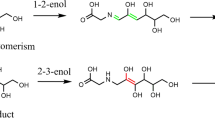
Three types of hydrogen abstraction from C3H8 by 3C2 radical have been considered. The prediction shows that the secondary hydrogen of C3H8 abstraction by 3C2 radical is the major pathway.








Similar content being viewed by others
References
Kruse T, Roth P (1999) Int J Chem Kinet 31:11–21
Mckellar AR (1960) J Astron Soc Can 54:97
Lambert DLM (1974) Bull Astron Inst Czech 25:216
Chaffee FH Jr, Lutz BL, Black JH, Vanden Bout PA, Snell RL (1980) Astrophys J 236:474
Danks AC, Lambert DL (1983) Astrophysics 124:188
Huang C, Zhu Z, Wang H, Pei L, Chen Y (2005) J Phys Chem A 109:3921
Huang C, Li Z, Zhao D, Xin Y, Pei L, Chen C, Chen Y (2004) Chin Sci Bull 49:438
Huang C, Zhu Z, Xin Y, Pei L, Chen C, Chen Y (2004) J Chem Phys 120:2225
Donnelly VM, Pasternack L (1979) Chem Phys 39:427–432
Reisler H, Mangir M, Wittig C (1979) J Chem Phys 71:2109–2117
Reisler H, Mangir M, Wittig C (1980) Chemical Physics 47:49–58
Pasternack L, Baronavski AP, McDonald JR (1980) J Chem Phys 73:3508–3510
Ballik EA, Ramsay DA (1959) J Chem Phys 31:1
Mangir MS, Reisler H, Wittig C (1980) J Chem Phys 73:829–835
Reisler H, Mangir MS, Wittig C (1980) J Chem Phys 73:2280–2286
Pasternack L, Pitts WM, McDonald JR (1981) Chem Phys 57:19–28
Pitts WM, Pasternack L, McDonald JR (1982) Chem Phys 68:417–422
Skell PS, Jackman LM, Ahmed S, McKee ML, Shevlin PB (1989) J Am Chem Soc 111:4422–4429
Gong M, Bao Y, Urdahl RS, Jackson WM (1994) Chem Phys Lett 217:210
Maluendes SA, McLean AD, Herbst E (1994) Chem Phys Lett 217:571–576
Horner DA, Curtiss LA, Gruen DM (1995) Chem Phys Lett 233:243–248
Blunt VM, Lin H, Sorkhabi O, Jackson WM (1996) Chem Phys Lett 257:347–350
Huang C, Zhao D, Pei L, Chen C, Chen Y (2004) Chem Phys Lett 389:230–235
Mebel AM, Kislov VV, Kaiser RI (2006) J Phys Chem A 110:2421–2433
Paramo A, Canosa A, Le Picard SD, Sims IR (2006) J Phys Chem A 110:3121–3127
Daugey N, Caubet P, Bergeat A, Costes M, Hickson KM (2008) Phys Chem Chem Phys 10:729–737
Paramo A, Canosa A, Le Picard SD, Sims IR (2008) J Phys Chem A 112:9591–9600
Hu R, Zhang Q, Chen Y (2010) J Chem Phys 132:164312–164317
Canosa A, Paramo A, Le Picard SD, Sims IR (2007) Icarus 187:558
Pasternack L, McDonald JR (1979) Chem Phys 43:173
Huo RP, Huang XR, Li JL, Zhang X, Sun CC (2012) Int J Quantum Chem 112:1078–1085
Li N, Huo RP, Zhang X, Huang XR, Li JL, Sun CC (2011) Chem Phys Lett 503:210–214
Huo RP, Zhang X, Huang XR, Li JL, Sun C C (2012) Mol Phys 1–13.
Schlegel HB (1986) J Chem Phys 84:4530
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154
Scuseria GE, Schaefer HF (1989) J Chem Phys 90:4
Pople JA, Gordon MH, Raghavachari K (1989) J Chem Phys 87:8
Lee TJ, Taylor PR (1989) Int J Quant Chem Symp 23:199
Rienstra-Kiracofe JC, Allen WD, Schaefer HF (2000) J Phys Chem A 104:9823
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, Revision D.02. Gaussian, Inc, Wallingford, CT
Corchado J C, Chuang Y Y, Fast P L, HuW P, Liu Y P, Lynch G C, Nguyen K A, Jackels C F, Ramos A F, Ellingson B A, Lynch B J, Zheng J, Melissas V S, Villàa J, Rossi I, Coitino E L, P Jingzhi, Albu T V (2007) Department of Chemistry and Supercomputing Institute; University ofMinnesota: Minneapolis, MN, Version 9.7
Liu YP, Lynch GC, Truong TN, Lu DH, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408
Garrett BC, Truhlar DG (1979) J Chem Phys 70:1593
Garrett BC, Truhlar DG (1979) J Am Chem Soc 101:4534
Neese F (2006) J Am Chem Soc 128:10213
Neese F. ORCA -an ab initio, density functional and semiempirical program package, Version 2.8, Bonn University.
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) J Comput Chem 25:1605
Hu WP, Rossi I, Corchado JC, Truhlar DG (1997) J Phys Chem A 101:6911
Gray HB (1973) Chemical Bonds. Benjamin, Menlo Park,CA
Hellwege KH, Hellwege AM (Eds.) (1976) Landolt-Bornstein: group II: Atomic and molecular physics vol 7: structure data of free polyatomic molecules. Springer, Berlin
Kuchitsu KE (1998) Structure of free polyatomic molecules - basic data. Springer, Berlin
Li JL, Gen CY, Huang XR, Sun CC (2006) J Chem Theory Comput 2:1551
Malick DKPGA, Montgomery JA (1998) J Chem Phys 108:5704
Huo RP, Zhang X, Huang XR, Li JL, Sun CC (2011) J Phys Chem A 115:3576–3582
Zheng J, Truhlar DG (2010) Phys Chem Chem Phys 12:7782
Acknowledgments
This work is supported by the National Natural Science Foundation of China (NSFC No. 21073075), Research Fund for the Doctoral Program of Higher Education of China (RFDP No. 20100061110046), the Special Funding of State Key Laboratory of Theoretical and Computational Chemistry, Jilin University and Basic Research Fund of Jilin University (No. 421010061439, 450060445067) and Graduate Innovation Fund of Jilin University (No.20121036). The authors are thankful for the reviewers’ invaluable comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huo, RP., Zhang, X., Huang, XR. et al. Direct ab initio study on the rate constants of radical C2(A3Πu) + C3H8 reaction. J Mol Model 19, 1009–1018 (2013). https://doi.org/10.1007/s00894-012-1616-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1616-8