Abstract.
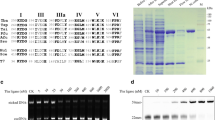
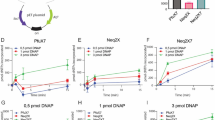
A DNA ligase gene from the hyperthermophilic bacterium Aquifex pyrophilus (Ap) was cloned and sequenced. An open reading frame of 2,157 bp that codes for a 82-kDa protein showed 40%–60% homology with a series of NAD+-dependent DNA ligases from different organisms. The recombinant enzyme Ap DNA ligase expressed in Escherichia coli was purified to homogeneity and characterized. The activity of Ap DNA ligase gradually increased in proportion to the concentration of monovalent salt up to 200 mM NaCl, 150 mM KCl, 200 mM NH4Cl, and 350 mM potassium glutamate. The optimum temperature and pH of Ap DNA ligase were greater than 65°C and 8.0–8.6, respectively, for nick-closing activity. More than 75% of the ligation activity was retained after incubation at 95°C for 60 min, whereas the half-lives of Thermus aquaticus and Escherichia coli DNA ligases at 95°C were ≤15 min and 5 min, respectively. Thermostable Ap DNA ligase was applied to repeat expansion detection (RED) and could be a useful enzyme in DNA diagnostics.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Lim, JH., Choi, J., Han, SJ. et al. Molecular cloning and characterization of thermostable DNA ligase from Aquifex pyrophilus, a hyperthermophilic bacterium. Extremophiles 5, 161–168 (2001). https://doi.org/10.1007/s007920100187
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s007920100187