Abstract
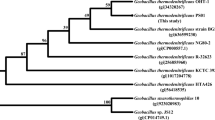
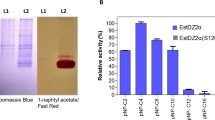
In the framework of the discovery of new thermophilic enzymes of potential biotechnological interest, we embarked in the characterization of a new thermophilic esterase from the thermophilic bacterium Geobacillus thermodenitrificans. The phylogenetic analysis of the GTNG_0744 esterase indicated that the sequence belongs to the enterochelin/enterobactin esterase group, which have never been recognized as a family in the lipases/esterase classification. These enzymes catalyze the last step in the acquisition of environmental Fe3+ through siderophore hydrolysis. In silico analysis revealed, for the first time, that the machinery for the uptake of siderophores is present in G. thermodenitrificans. The purified recombinant enzyme, EstGtA3, showed different substrate specificity from known enterochelin/enterobactin esterases, recognizing short chain esters with a higher specificity constant for 4-NP caprylate. The enzyme does not require cofactors for its activity, is active in the pH range 7.0–8.5, has highest activity at 60 °C and is 100% stable when incubated for 16 h at 55 °C. DTT, β-mercaptoethanol and Triton X-100 have an activating effect on the enzymatic activity. Organic solvents have in general a negative effect on the enzyme, but n-hexane is a strong activator up to 150, making EstGtA3 a good candidate for applications in biotechnology.





Similar content being viewed by others
Abbreviations
- 4-NP:
-
4-nitrophenyl
- 4-NPC12:
-
4-nitrophenyl laurate
- 4-NPC2:
-
4-nitrophenyl acetate
- 4-NPC4:
-
4-nitrophenyl butyrate
- 4-NPC8:
-
4-nitrophenyl caprylate
- DTT:
-
Dithiothreitol
- DMF:
-
Dimethylformamide
- DMSO:
-
Dimethyl sulfoxide
- EDTA:
-
Ethylenediaminetetraacetic acid
- EstGtA3:
-
esterase from G. thermodenitrificans NG80-2
- EtOH:
-
Ethanol
- MeOH:
-
Methanol
- MES:
-
2-(N-morpholino)ethanesulfonic acid
- PMSF:
-
phenylmethane sulfonyl fluoride
- SDS–PAGE:
-
Sodium Dodecyl Sulphate - PolyAcrylamide Gel Electrophoresis
- SDS:
-
Sodium Dodecyl Sulphate
- TCV:
-
inhibitor tris-catechol vector
References
Abergel RJ, Zawadzka AM, Hoette TM, Raymond KN (2009) Enzymatic hydrolysis of trilactone siderophores: where chiral recognition occurs in enterobactin and bacillibactin iron transport. J Am Chem Soc 131:12682–12692. https://doi.org/10.1021/ja903051q
Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. https://doi.org/10.1093/bioinformatics/bti770
Arpigny JL, Jaeger KE (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343(Pt 1):177–183
Bornscheuer UT (2002) Microbial carboxyl esterases: classification, properties and application in biocatalysis. Fems Microbiol Rev 26:73–81. https://doi.org/10.1111/j.1574-6976.2002.tb00599.x
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Castilla A, Panizza P, Rodriguez D, Bonino L, Diaz P, Irazoqui G, Rodriguez Giordano S (2017) A novel thermophilic and halophilic esterase from Janibacter sp. R02, the first member of a new lipase family (Family XVII). Enzyme Microb Technol 98:86–95. https://doi.org/10.1016/j.enzmictec.2016.12.010
Charbonneau DM, Meddeb-Mouelhi F, Beauregard M (2010) A novel thermostable carboxylesterase from Geobacillus thermodenitrificans: evidence for a new carboxylesterase family. J Biochem 148:299–308. https://doi.org/10.1093/jb/mvq064
Chenault SS, Earhart CF (1991) Organization of genes encoding membrane proteins of the Escherichia coli ferrienterobactin permease. Mol Microbiol 5:1405–1413
Cobucci-Ponzano B, Aurilia V, Riccio G, Henrissat B, Coutinho PM, Strazzulli A, Padula A, Corsaro MM, Pieretti G, Pocsfalvi G, Fiume I, Cannio R, Rossi M, Moracci M (2010) A New Archaeal beta-Glycosidase from Sulfolobus solfataricus seeding a novel retaining beta-glycan-specific glycoside hydrolase family along with the human non-lysosomal glucosylceramidase gba2. J Biol Chem 285:20691–20703. https://doi.org/10.1074/jbc.M109.086470
Cobucci-Ponzano B, Conte F, Strazzulli A, Capasso C, Fiume I, Pocsfalvi G, Rossi M, Moracci M (2010) The molecular characterization of a novel GH38 alpha-mannosidase from the crenarchaeon Sulfolobus solfataricus revealed its ability in de-mannosylating glycoproteins. Biochimie 92:1895–1907. https://doi.org/10.1016/j.biochi.2010.07.016
Cobucci-Ponzano B, Zorzetti C, Strazzulli A, Carillo S, Bedini E, Corsaro MM, Comfort DA, Kelly RM, Rossi M, Moracci M (2011) A novel alpha-d-galactosynthase from Thermotoga maritima converts beta-d-galactopyranosyl azide to alpha-galacto-oligosaccharides. Glycobiology 21:448–456. https://doi.org/10.1093/glycob/cwq177
Cobucci-Ponzano B, Perugino G, Strazzulli A, Rossi M, Moracci M (2012) Thermophilic glycosynthases for oligosaccharides synthesis. Methods Enzymol 510:273–300. https://doi.org/10.1016/B978-0-12-415931-0.00015-X
Cobucci-Ponzano B, Strazzulli A, Iacono R, Masturzo G, Giglio R, Rossi M, Moracci M (2015) Novel thermophilic hemicellulases for the conversion of lignocellulose for second generation biorefineries. Enzyme Microb Tech doi:10.1016/j.enzmictec.2015.06.014
De Santi C, Tedesco P, Ambrosino L, Altermark B, Willassen NP, de Pascale D (2014) A new alkaliphilic cold-active esterase from the psychrophilic marine bacterium Rhodococcus sp.: functional and structural studies and biotechnological potential. Appl Biochem Biotechnol. 172:3054–3068. https://doi.org/10.1007/s12010-013-0713-1
Elleuche S, Schäfers C, Blank S, Schröder C, Antranikian G (2015) Exploration of extremophiles for high temperature biotechnological processes. Curr Opin Microbiol 25:113–119. https://doi.org/10.1016/j.mib.2015.05.011
Huang J, Zhang Y, Hu Y (2016) Functional Characterization of a Marine Bacillus Esterase and its Utilization in the Stereo-Selective Production of D-Methyl Lactate. Appl Biochem Biotechnol 180:1467–1481. https://doi.org/10.1007/s12010-016-2180-y
Iacono R, Cobucci-Ponzano B, Strazzulli A, Giglio R, Maurelli L, Moracci M (2016) (Hyper)thermophilic biocatalysts for second generation biorefineries. Chem Today 34:4
Iacono R, Strazzulli A, Maurelli L, Curci N, Casillo A, Corsaro MM, Moracci M, Cobucci-Ponzano B (2019) GlcNAc De-N-Acetylase from the Hyperthermophilic Archaeon Sulfolobus solfataricus. Applied and Environmental Microbiology 85 doi:10.1128/AEM.01879–18
Jaeger KE, Eggert T (2002) Lipases for biotechnology. Curr Opin Biotechnol 13:390–397
Jain I, Kumar V, Satyanarayana T (2014) Applicability of recombinant beta-xylosidase from the extremely thermophilic bacterium Geobacillus thermodenitrificans in synthesizing alkylxylosides. Bioresour Technol 170:462–469. https://doi.org/10.1016/j.biortech.2014.07.113
Jayanath G, Mohandas SP, Kachiprath B, Solomon S, Sajeevan TP, Bright Singh IS, Philip R (2018) A novel solvent tolerant esterase of GDSGG motif subfamily from solar saltern through metagenomic approach: Recombinant expression and characterization. Int J Biol Macromol 119:393–401. https://doi.org/10.1016/j.ijbiomac.2018.06.057
Kanamori Y, Watanabe M, Kawauchi K, Chen Y-G, Yanagishita H, Hirata H (2005) Kinetic Resolution of Enantiomers in Racemic and Enantiomerically Enriched 2-Alkanols by Pseudomonas cepacia Lipase Catalyzed Transesterification with Isopropenyl Acetate in Organic Solvent vol 54. doi:10.5650/jos.54.21,
Khan A, Singh P, Srivastava A (2018) Synthesis, nature and utility of universal iron chelator—Siderophore: a review. Microbiol Res 212–213:103–111. https://doi.org/10.1016/j.micres.2017.10.012
Kim J, Kim S, Yoon S, Hong E, Ryu Y (2015) Improved enantioselectivity of thermostable esterase from Archaeoglobus fulgidus toward (S)-ketoprofen ethyl ester by directed evolution and characterization of mutant esterases. Appl Microbiol Biotechnol 99:6293–6301. https://doi.org/10.1007/s00253-015-6422-7
Kumagai PS, Gutierrez RF, Lopes JLS, Martins JM, Jameson D, Castro AM, Martins LF, DeMarco R, Bossolan NRS, Wallace BA, Araujo APU (2018) Characterization of esterase activity from an Acetomicrobium hydrogeniformans enzyme with high structural stability in extreme conditions. Extremophiles: Life Under Extreme Conditions 22:781–793. https://doi.org/10.1007/s00792-018-1038-3
Kumar S, Stecher G, Tamura K (2016) MEGA7: Molecular evolutionary genetics analysis Version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. https://doi.org/10.1093/molbev/msw054
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck—a Program to Check the Stereochemical Quality of Protein Structures. J Appl Crystallogr 26:283–291. https://doi.org/10.1107/S0021889892009944
Levisson M, van der Oost J, Kengen SWM (2007) Characterization and structural modeling of a new type of thermostable esterase from Thermotoga maritima. Febs J 274:2832–2842. https://doi.org/10.1111/j.1742-4658.2007.05817.x
Levisson M, van der Oost J, Kengen SWM (2009) Carboxylic ester hydrolases from hyperthermophiles. Extremophiles: Life Under Extreme Conditions 13:567–581. https://doi.org/10.1007/s00792-009-0260-4
Li WQ, Shi H, Ding HH, Wang LL, Zhang Y, Li X, Wang F (2018) Characterization of two novel thermostable esterases from Thermoanaerobacterium thermosaccharolyticum. Protein Expres Purif 152:64–70. https://doi.org/10.1016/j.pep.2018.04.010
Li X, Yu H (2015) Characterization of an organic solvent-tolerant lipase from Haloarcula sp. G41 and its application for biodiesel production. Folia Microbiol 59(6):455–63. doi: 10.1007/s12223–014–0320–8
Lin H, Fischbach MA, Liu DR, Walsh CT (2005) In vitro characterization of salmochelin and enterobactin trilactone hydrolases IroD, IroE, and Fes. J Am Chem Soc 127:11075–11084. https://doi.org/10.1021/ja0522027
López-López O, Cerdán ME, González Siso MI (2014) New extremophilic lipases and esterases from metagenomics. Curr Protein Pept Sc 15:445–455
Manachini PL, Mora D, Nicastro G, Parini C, Stackebrandt E, Pukall R, Fortina MG (2000) Bacillus thermodenitrificans sp. nov., nom. rev. International journal of systematic and evolutionary microbiology 50 Pt 3:1331–1337 doi:10.1099/00207713–50–3–1331
Marcolongo L, La Cara F, Morana A, Di Salle A, Del Monaco G, Paixao SM, Alves L, Ionata E (2015) Properties of an alkali-thermo stable xylanase from Geobacillus thermodenitrificans A333 and applicability in xylooligosaccharides generation. World J Microbiol Biotechnol 31:633–648. https://doi.org/10.1007/s11274-015-1818-1
Nazina TN, Tourova TP, Poltaraus AB, Novikova EV, Grigoryan AA, Ivanova AE, Lysenko AM, Petrunyaka VV, Osipov GA, Belyaev SS, Ivanov MV (2001) Taxonomic study of aerobic thermophilic bacilli: descriptions of Geobacillus subterraneus gen. nov., sp. nov. and Geobacillus uzenensis sp. nov. from petroleum reservoirs and transfer of Bacillus stearothermophilus, Bacillus thermocatenulatus, Bacillus thermoleovorans, Bacillus kaustophilus, Bacillus thermodenitrificans to Geobacillus as the new combinations G. stearothermophilus, G. th. Int J Syst Evolut Microbiol 51:433–446. https://doi.org/10.1099/00207713-51-2-433
Ollinger J, Song KB, Antelmann H, Hecker M, Helmann JD (2006) Role of the Fur regulon in iron transport in Bacillus subtilis. J Bacteriol 188:3664–3673. https://doi.org/10.1128/JB.188.10.3664-3673.2006
Pereira MR, Maester TC, Mercaldi GF, de Macedo Lemos EG, Hyvonen M, Balan A (2017) From a metagenomic source to a high-resolution structure of a novel alkaline esterase. Appl Microbiol Biotechnol 101:4935–4949. https://doi.org/10.1007/s00253-017-8226-4
Perraud Q, Moynie L, Gasser V, Munier M, Godet J, Hoegy F, Mely Y, Mislin GLA, Naismith JH, Schalk IJ (2018) A Key Role for the Periplasmic PfeE Esterase in Iron Acquisition via the Siderophore Enterobactin in Pseudomonas aeruginosa. Acs Chem Biol 13:2603–2614. https://doi.org/10.1021/acschembio.8b00543
Ramnath L, Sithole B, Govinden R (2017) Classification of lipolytic enzymes and their biotechnological applications in the pulping industry. Can J Microbiol 63:179–192. https://doi.org/10.1139/cjm-2016-0447
Ranjan R, Yadav MK, Suneja G, Sharma R (2018) Discovery of a diverse set of esterases from hot spring microbial mat and sea sediment metagenomes. Int J Biol Macromol 119:572–581. https://doi.org/10.1016/j.ijbiomac.2018.07.170
Samoylova YV, Sorokina KN, Romanenko MV, Parmon VN (2018) Cloning, expression and characterization of the esterase estUT1 from Ureibacillus thermosphaericus which belongs to a new lipase family XVIII. Extremophiles : life under extreme conditions 22:271–285. https://doi.org/10.1007/s00792-018-0996-9
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Soding J, Thompson JD, Higgins DG (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Sirec T, Strazzulli A, Isticato R, De Felice M, Moracci M, Ricca E (2012) Adsorption of β-galactosidase of Alicyclobacillus acidocaldarius on wild type and mutants spores of Bacillus subtilis. Microb Cell Fact 11:100. https://doi.org/10.1186/1475-2859-11-100
Strazzulli A, Cobucci-Ponzano B, Carillo S, Bedini E, Corsaro MM, Pocsfalvi G, Withers SG, Rossi M, Moracci M (2017) Introducing transgalactosylation activity into a family 42 beta-galactosidase. Glycobiology 27:425–437. https://doi.org/10.1093/glycob/cwx013
Strazzulli A, Fusco S, Cobucci-Ponzano B, Moracci M, Contursi P (2017) Metagenomics of microbial and viral life in terrestrial geothermal environments. Rev Environ Sci Bio 16:425–454. https://doi.org/10.1007/s11157-017-9435-0
Strazzulli A, Iacono R, Giglio R, Moracci M, Cobucci-Ponzano B (2017c) Metagenomics of hyperthermophilic environments: Biodiversity and biotechnology. In: Microbial Ecology of Extreme Environments. pp 103–135. doi:10.1007/978–3–319–51686–8_5
Wang L, Tang Y, Wang S, Liu RL, Liu MZ, Zhang Y, Liang FL, Feng L (2006) Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles : life under extreme conditions 10:347–356. https://doi.org/10.1007/s00792-006-0505-4
Yang Z, Zhang Y, Shen T, Xie Y, Mao Y, Ji C (2013) Cloning, expression and biochemical characterization of a novel, moderately thermostable GDSL family esterase from Geobacillus thermodenitrificans T2. J Biosci Bioeng 115:133–137. https://doi.org/10.1016/j.jbiosc.2012.08.016
Yang X, Wu L, Xu Y, Ke C, Hu F, Xiao X, Huang J (2018) Identification and characterization of a novel alkalistable and salt-tolerant esterase from the deep-sea hydrothermal vent of the East Pacific Rise. Microbiologyopen 7:e00601. https://doi.org/10.1002/mbo3.601
Yu N, Yang JC, Yin GT, Li RS, Zou WT, He C (2018) Identification and characterization of a novel esterase from Thauera sp. Biotechnol Appl Bioc 65:748–755. https://doi.org/10.1002/bab.1659
Zhang J, Zhao M, Yu D, Yin J, Zhang H, Huang X (2017) Biochemical characterization of an enantioselective esterase from Brevundimonas sp. LY-2. Microb Cell Fact 16:112 doi:10.1186/s12934-017-0727-4
Zhu Y, Li J, Cai H, Ni H, Xiao A, Hou L (2013) Characterization of a new and thermostable esterase from a metagenomic library. Microbiol Res 168:589–597. https://doi.org/10.1016/j.micres.2013.04.004
Zhu Y, Zheng W, Ni H, Liu H, Xiao A, Cai H (2015) Molecular cloning and characterization of a new and highly thermostable esterase from Geobacillus sp. JM6. J Basic Microbiol 55:1219–1231. https://doi.org/10.1002/jobm.201500081
Acknowledgements
We thank Francesco La Cara and collaborators at the Research Institute on Terrestrial Ecosystems (IRET) from the National Research Council of Italy for the gift of Geobacillus thermodenitrificans NG80-2 genome. We are grateful to Chiara Nobile and Marco Petruzziello at the Institute of Biosciences and BioResources (IBBR) from the National Research Council of Italy for administrative and technical assistance. This work was supported by a grant from the Italian Ministry of Research (MIUR) PON03PE_00107_1 BIOPOLIS.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Atomi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nicola Curci and Andrea Strazzulli contributed equally to the work
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Curci, N., Strazzulli, A., De Lise, F. et al. Identification of a novel esterase from the thermophilic bacterium Geobacillus thermodenitrificans NG80-2. Extremophiles 23, 407–419 (2019). https://doi.org/10.1007/s00792-019-01093-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-019-01093-9