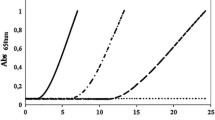
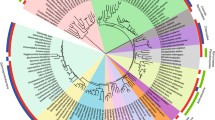
Abstract
Protein disulfide oxidoreductases (PDOs) are proteins involved in disulfide bond formation playing a crucial role in adaptation to extreme environment. This paper reports the functional and structural characterization of Sso1120, a PDO from the hyperthermophilic archaeon Sulfolobus solfataricus. The protein was expressed in Escherichia coli and purified to homogeneity. The functional characterization showed that the enzyme has reductase activity, as tested by insulin assay, but differently from the other PDOs, it does not present isomerase activity. In addition it is able to form a redox couple with the thioredoxin reductase that could be used in undiscovered pathways. The protein revealed a melting point of around 90 °C in CD spectroscopy-monitored thermal denaturation and high denaturant resistance. The X-ray crystallographic structure was solved at 1.80 Å resolution, showing differences with respect to other PDOs and an unexpected similarity with the N-terminal domain of the alkyl hydroperoxide reductase F component from Salmonella typhimurium. On the basis of the reported data and of bioinformatics and phylogenetic analyses, a possible involvement of this atypical PDO in a new antioxidant system of S. solfataricus has been proposed.







Similar content being viewed by others
References
Beeby M, O’Connor BD, Ryttersgaard C, Boutz DR, Perry LJ, Yeates TO (2005) The genomics of disulfide bonding and protein stabilization in thermophiles. PLoS Biol 3:e309. doi:10.1371/journal.pbio.0030309
Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL (1998) Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta Crystallogr D54:905–921. doi:10.1107/S0907444998003254
Contursi P, Jensen S, Aucelli T, Rossi M, Bartolucci S, She Q (2006) Characterization of the Sulfolobus host-SSV2 virus interaction. Extremophiles 10:615–627
Contursi P, D’Ambrosio K, Pirone L, Pedone E, Aucelli T, She Q, De Simone G, Bartolucci S (2011) C68 from the Sulfolobus islandicus plasmid-virus pSSVx is a novel member of the AbrB-like transcription factor family. Biochem J 435:157–166
Cudney R, Patel S, Weisgraber K, Newhouse Y, McPherson A (1994) Screening and optimization strategies for macromolecular crystal growth. Acta Crystallogr D50:414–423. doi:10.1107/S0907444994002660
D’Ambrosio K, Pedone E, Langella E, De Simone G, Rossi M, Pedone C, Bartolucci S (2006) A novel member of the protein disulfide oxidoreductase family from Aeropyrum pernix K1: structure, function and electrostatics. J Mol Biol 362:743–752. doi:10.1016/j.jmb.2006.07.038
Gruber CW, Cemazar M, Heras B, Martin JL, Craik DJ (2006) Protein disulfide isomerase: the structure of oxidative folding. Trends Biochem Sci 31:455–464. doi:10.1016/j.tibs.2006.06.001
Guagliardi A, de Pascale D, Cannio R, Nobile V, Bartolucci S, Rossi M (1995) The purification, cloning, and high level expression of a glutaredoxin-like protein from the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 270:5748–5755
Holm L, Rosenstrom P (2010) Dali server: conservation mapping in 3D. Nucleic Acids Res 38:W545–W549. doi:10.1093/Nar/Gkq366
Jancarik J, Kim SH (1991) Sparse-matrix dampling - a screening method for crystallization of proteins. J Appl Cryst 24:409–411. doi:10.1107/S0021889891004430
Jones TA, Zou JY, Cowan SW, Kjeldgaard M (1991) Improved methods for building protein models in electron-density maps and the location of errors in these models. Acta Crystallogr A 47:110–119. doi:10.1107/S0108767390010224
Koshkin A, Knudsen GM, de Montellano PRO (2004) Intermolecular interactions in the AhpC/AhpD antioxidant defense system of Mycobacterium tuberculosis. Arch Biochem Biophys 427:41–47. doi:10.1016/j.abb.2004.04.017
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Laskowski RA, Macarthur MW, Moss DS, Thornton JM (1993) Procheck: a program to check the stereochemical quality of protein structures. J Appl Crystallogr 26:283–291. doi:10.1107/S0021889892009944
Limauro D, Pedone E, Pirone L, Bartolucci S (2006) Identification and characterization of 1-Cys peroxiredoxin from Sulfolobus solfataricus and its involvement in the response to oxidative stress. FEBS J 273:721–731. doi:10.1111/j.1742-4658.2006.05104.x
Limauro D, Pedone E, Galdi I, Bartolucci S (2008) Peroxiredoxins as cellular guardians in Sulfolobus solfataricus: characterization of Bcp1, Bcp3 and Bcp4. FEBS J 275:2067–2077. doi:10.1111/j.1742-4658.2008.06361.x
Limauro D, Saviano M, Galdi I, Rossi M, Bartolucci S, Pedone E (2009) Sulfolobus solfataricus protein disulphide oxidoreductase: insight into the roles of its redox sites. Protein Eng Des Sel 22:19–26. doi:10.1093/protein/gzn061
Limauro D, D’Ambrosio K, Langella E, De Simone G, Galdi I, Pedone C, Pedone E, Bartolucci S (2010) Exploring the catalytic mechanism of the first dimeric Bcp: functional, structural and docking analyses of Bcp4 from Sulfolobus solfataricus. Biochimie 92:1435–1444. doi:10.1016/j.biochi.2010.07.006
Marino SM, Gladyshev VN (2011) Redox biology: computational approaches to the investigation of functional cysteine residues. Antioxid Redox Signal 15:135–146. doi:10.1089/ars 2010.3561
McPherson A (1990) Current approaches to macromolecular crystallization. Eur J Biochem 189:1–23. doi:10.1111/j.1432-1033.1990.tb15454.x
Myers JK, Pace CN, Scholtz JM (1995) Denaturant M-values and heat-capacity changes: relation to changes in accessible surface-areas of protein unfolding. Protein Sci 4:2138–2148
Navaza J (1994) Amore: an automated package for molecular replacement. Acta Crystallogr A 50:157–163. doi:10.1107/S0108767393007597
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Method Enzymol 276:307–326. doi:10.1016/S0076-6879(97)76066-X
Pedone E, Saviano M, Rossi M, Bartolucci S (2001) A single point mutation (Glu85Arg) increases the stability of the thioredoxin from Escherichia coli. Protein Eng 14:255–260
Pedone E, Ren B, Ladenstein R, Rossi M, Bartolucci S (2004) Functional properties of the protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus: a member of a novel protein family related to protein disulfide-isomerase. Eur J Biochem 271:3437–3448. doi:10.1111/j.0014-2956.2004.04282
Pedone E, D’Ambrosio K, De Simone G, Rossi M, Pedone C, Bartolucci S (2006a) Insights on a new PDI-like family: structural and functional analysis of a protein disulfide oxidoreductase from the bacterium Aquifex aeolicus. J Mol Biol 356:155–164. doi:10.1016/j.jmb.2005.11.041
Pedone E, Limauro D, D’Alterio R, Rossi M, Bartolucci S (2006b) Characterization of a multifunctional protein disulfide oxidoreductase from Sulfolobus solfataricus. FEBS J 273:5407–5420. doi:10.1111/j.1742-4658.2006.05533.x
Pedone E, Limauro D, Bartolucci S (2008) The machinery for oxidative protein folding in thermophiles. Antioxid Redox Signal 10:157–169. doi:10.1089/ars 2007.1855
Pedone E, Limauro D, D’Ambrosio K, De Simone G, Bartolucci S (2010) Multiple catalytically active thioredoxin folds: a winning strategy for many functions. Cell Mol Life Sci 67:3797–3814. doi:10.1007/s00018-010-0449-9
Ren B, Tibbelin G, de Pascale D, Rossi M, Bartolucci S, Ladenstein R (1998) A protein disulfide oxidoreductase from the archaeon Pyrococcus furiosus contains two thioredoxin fold units. Nat Struct Biol 5:602–611. doi:10.1038/862
Reynolds CM, Poole LB (2000) Attachment of the N-terminal domain of Salmonella typhimurium AhpF to Escherichia coli thioredoxin reductase confers AhpC reductase activity but does not affect thioredoxin reductase activity. Biochemistry 39:8859–8869. doi:10.1021/Bi000826d
Roberts BR, Wood ZA, Jonsson TJ, Poole LB, Karplus PA (2005) Oxidized and synchrotron cleaved structures of the disulfide redox center in the N-terminal domain of Salmonella typhimurium AhpF. Protein Sci 14:2414–2420. doi:10.1110/Ps.051459705
Sato Y, Inaba K (2012) Disulfide bond formation network in the three biological kingdoms, bacteria, fungi and mammals. FEBS J 279:2262–2271. doi:10.1111/j.1742-4658.2012.08593.x
Acknowledgments
We gratefully acknowledge the support from MIUR for project FIRB no. RBRN07BMCT and Università Federico II per l’Avvio di Ricerche Originali (FARO 2011) CUP:E61J1200060005.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Albers.
D. Limauro and G. De Simone contributed equally to this work.
Rights and permissions
About this article
Cite this article
Limauro, D., De Simone, G., Pirone, L. et al. Sulfolobus solfataricus thiol redox puzzle: characterization of an atypical protein disulfide oxidoreductase. Extremophiles 18, 219–228 (2014). https://doi.org/10.1007/s00792-013-0607-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0607-8