Abstract.
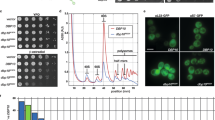
The hyperthermophilic archaeon, Pyrococcus furiosus, expresses a small, α-crystallin-like protein in response to exposure to extreme temperatures, above 103°C. The P. furiosus small heat shock protein (Pfu-sHSP) forms large oligomeric complexes. Based on the available crystal structures of the Methanocaldococcus jannaschii and wheat sHSPs, the protruding carboxy terminal domain is probably involved in subunit interactions. We constructed Pfu-sHSP mutants to analyze chaperone function and to study multi-subunit assembly. The results confirmed that the carboxy terminus of Pfu-sHSP is involved in inter-dimer interactions, whereas the amino terminal deletion mutant still exhibited the wild-type assembly characteristics. The ability to form oligomeric complexes via the carboxy terminal domain was shown to be necessary for thermotolerance of Escherichia coli overexpressing Pfu-sHSP. The amino terminal domain was not required for inter-species thermotolerance.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Laksanalamai, .P., Jiemjit, .A., Bu, .Z. et al. Multi-subunit assembly of the Pyrococcus furiosus small heat shock protein is essential for cellular protection at high temperature. Extremophiles 7, 79–83 (2003). https://doi.org/10.1007/s00792-002-0298-z
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00792-002-0298-z