Abstract
Objectives
To compare selective COX-2 inhibitors with ibuprofen in terms of analgesia, rescue medication consumption, and adverse effects after impacted third molar removal.
Materials and methods
Electronic databases were searched. Single dose, double-blind, randomized, and controlled clinical trials comparing the analgesic effect of a selective COX-2 inhibitor versus at least one active control group using ibuprofen after impacted third molar removal were selected.
Results
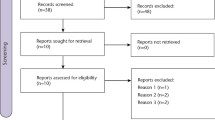
Twelve studies were included for the qualitative synthesis and eight were included in the meta-analysis. No statistically significant differences were found between selective COX-2 inhibitors and ibuprofen in terms of pain relief after 6, 8, and 12 h. Rescue analgesia use after 24 h was significantly greater in the ibuprofen group than in the selective COX-2 inhibitor group. There were no statistically significant differences in the number of patients presenting one or more adverse events between the two groups, though ibuprofen intake was related with more nausea and vomiting.
Conclusions
No statistically significant differences were found in terms of pain relief 6, 8, and 12 h post-medication between selective COX-2 inhibitors and ibuprofen following totally or partially impacted third molar removal. The patients who consumed selective COX-2 inhibitors needed less rescue analgesia after 24 h. The occurrence of one or more adverse events was similar in both groups, though patients who consumed ibuprofen had more nausea and vomiting.
Clinical relevance
COX-2 inhibitors could be considered a suitable alternative to ibuprofen for pain relief after third molar extraction in patients at risk of developing nausea and vomiting. Also, COX-2 inhibitors seem to slightly reduce the need of rescue medication consumption.







Similar content being viewed by others
References
Moore A, Makinson G, Li C (2013) Patient-level pooled analysis of adjudicated gastrointestinal outcomes in celecoxib clinical trials: meta-analysis of 51,000 patients enrolled in 52 randomized trials. Arthritis Res Ther 15:R6
Jarupongprapa S, Ussavasodhi P, Katchamart W (2013) Comparison of gastrointestinal adverse effects between cyclooxygenase-2 inhibitors and non-selective, non-steroidal anti-inflammatory drugs plus proton pump inhibitors: a systematic review and meta-analysis. J Gastroenterol 48:830–838
McGettigan P, Henry D (2006) Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA 296:1633–1644
Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, Lines C, Riddell R, Morton D, Lanas A, Konstam MA, Baron JA (2005) Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med 352:1092–1102
De Vecchis R, Baldi C, Di Biase G, Ariano C, Cioppa C, Giasi A, Valente L, Cantatrione S (2014) Cardiovascular risk associated with celecoxib or etoricoxib: a meta-analysis of randomized controlled trials which adopted comparison with placebo or naproxen. Minerva Cardioangiol 62:437–448
Ungprasert P, Srivali N, Kittanamongkolchai W (2015) Non-steroidal anti-inflammatory drugs and risk of heart failure exacerbation: a systematic review and meta-analysis. Eur J Intern Med 26:685–690
Mackenzie IS, Wei L, MacDonald TM (2013) Cardiovascular safety of lumiracoxib: a meta-analysis of randomised controlled trials in patients with osteoartritis. Eur J Clin Pharmacol 69:133–141
Coxib and traditional NSAID Trialists’ (CNT) Collaboration (2013) Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet 382:769–779
Derry S, Moore RA (2013) Single dose oral celecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev 10:CD004233
Bulley S, Derry S, Moore RA, McQuay HJ (2009) Single dose oral rofecoxib for acute postoperative pain in adults. Cochrane Database Syst Rev 4:CD004604
Clarke R, Derry S, Moore RA (2014) Single dose oral etoricoxib for acute postoperative pain in adults. Cochrane Database Syst Rev 5:CD004309
Roy YM, Derry S, Moore RA (2010) Single dose oral lumiracoxib for postoperative pain in adults. Cochrane Database Syst Rev 7:CD006865
Tirunagari SK, Derry S, Moore RA, McQuay HJ (2009) Single dose oral etodolac for acute postoperative pain in adults. Cochrane Database Syst Rev 3:CD007357
Silva RCL, Riera R, Saconato H (2011) Lumiracoxib for acute postoperative dental pain: a systematic review of randomized clinical trials. Sao Paulo Med J 129:335–345
Moher D, Liberati A, Tetzlaff J, Altman DG, Group, P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Moore A, McQuay H, Gavaghan D (1997) Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics: verification from independent data. Pain 69:127–130
Clinicaltrials.gov. https://clinicaltrials.gov. Accessed 28 November 2017
OpenGrey. http://opengrey.eu. Accessed 28 November 2017
International Clinical Trials Registry Platform (ICTRP). World Health Organization. http://www.who.int/ictrp/en/. Accessed 28 November 2017
Higgins JPT, Green S, editors. (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane- handbook.org. Accessed 15 November 2017
Gupta SK (2011) Intention-to-treat concept: a review. Perspect Clin Res 2:109–112 http://www.ncbi.nlm.nih.gov/pubmed/21897887. Accessed 21 Oct 2017
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Caldwell DM, Ades AE, Higgins JPT (2005) Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 331:897–900 http://www.ncbi.nlm.nih.gov/pubmed/16223826. Accessed 21 Oct 2017
Doyle G, Jayawardena S, Ashraf E, Cooper SA (2002) Efficacy and tolerability of nonprescription ibuprofen versus celecoxib for dental pain. J Clin Pharmacol 42:912–919
Brown JD, Daniels SE, Bandy DP, Ko AT, Gammaitoni A, Mehta A, Boice JA, Losada MC, Peloso PM (2013) Evaluation of multiday analgesia with etoricoxib in a double-blind, randomized controlled trial using the postoperative third-molar extraction dental pain model. Clin J Pain 29:492–498
Khan AA, Brahim JS, Rowan JS, Dionne RA (2002) In vivo selectivity of a selective cyclooxyrgenase 2 inhibitor in the oral surgery model. Cain Pharmacy Ther 72:44–49
Erich E, Dallo A, De Repeliere I, Van Hecke A, Riendeau D, Yuan W, Porras A, Wittreich A, Seibold JR, De Schepper P, Mehlisch DR, Gertz BJ (1999) Characterization of rofecoxib as a cyclooxygenase-2 isoform inhibitor and demonstration of analgesia in the dental pain model. Clin Pharmacy Ther 65:336–347
Chiu WK, Cheung LK (2005) Efficacy of preoperative oral rofecoxib in pain control for third molar surgery. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 99:47–53
Morse Z, Trump A, Kevelham E (2006) Ibuprofen as a pre-emptive analgesic is as effective as rofecoxib for mandibular third molar surgery. Odontology 94:59–63
Albuquerque AFM, Fonteles CSR, do Val DR, Chaves HV, Bezerra MM, Pereira KMA, de Barros Silva PG, de Lima BB, Soares ECS, Ribeiro TR, Costa FWG (2017) Effect of pre-emptive analgesia on clinical parameters and tissue levels of TNF-a and IL-1b in third molar surgery: a triple-blind, randomized, placebo-controlled study. Int J Oral Maxillofac Surg 46:1615–1625
Al-Sukhun J, Al-Sukhun S, Penttila H, Ashammakhi N, Al-Sukhun R (2012) Preemptive analgesic effect of low doses of celecoxib is superior to low doses of traditional nonsteroidal anti-inflammatory drugs. J Craniofac Surg 23:526–529
Daniels SE, Brandy DP, Christensen SE, Boice J, Losada MC, Liu H, Mehta A, Peloso PM (2011) Evaluation of the dose range of etoricoxib in an acute pain setting using the postoperative dental pain model. Clin J Pain 27:1–8
Cheung R, Krishnaswami S, Kowalski K (2007) Efficacy of celecoxib in postoperative oral surgery pain: a single-dose, two-center, randomized, double-blind, active- and placebo-controlled study. Clin Ther 29:2498–2510
Malmstrom K, Sapre A, Coughlin H, Agrawal NGB, Mazenko RS, Fricke JR (2004) Etoricoxib in acute pain associated with dental surgery: a randomized, double-blind, placebo- and active comparator-controlled dose-ranging study. Clin Ther 26:667–679
Malmstrom K, Fricke JR, Kotey P, Kress B, Morrison B (2002) A comparison of rofecoxib versus celecoxib in treating pain after dental surgery: a single-center, randomized, double-blind, placebo- and active-comparator-controlled, parallel-group, single-dose study using the dental impaction pain model. Clin Ther 24:1549–1560
Malmstrom K, Daniels S, Kotey P, Seidenberg BC, Desjardins PJ (1999) Comparison of rofecoxib and celecoxib, two cyclooxygenase-2 inhibitors, in post- operative dental pain: a randomized, placebo- and active-comparator controlled clinical trial. Clin Ther 21:1653–1663
Morrison BW, Christensen S, Yuan W, Brown J, Amlani S, Seidenberg B (1999) Analgesic efficacy of the cyclooxygenase-2-specific inhibitor rofecoxib in post-dental surgery pain: a randomized, controlled trial. Clin Ther 21:943–953
Zelenakas K, Fricke JR, Jayawardene S, Kellstein D (2004) Analgesic efficacy of single oral doses of lumiracoxib and ibuprofen in patients with postoperative dental pain. Int J Clin Pract 58:251–256
McQuay H, Carroll D, Moore A (1996) Variation in the placebo effect in randomised controlled trials of analgesics: all is as blind as it seems. Pain 64:331–335
Moore A, McQuay H, Gavaghan D (1996) Deriving dichotomous outcome measures from continuous data in randomised controlled trials of analgesics. Pain 66:229–237
Morales DR, Lipwirth BJ, Guthrie B, Jackson C, Donnan PT, Santiago VH (2014) Safety risks for patients with aspirin-exacerbated respiratory disease after acute exposure to selective nonsteroidal anti-inflammatory drugs and COX-2 inhibitors: meta-analysis of controlled clinical trials. J Allergy Clin Immunol 134:40–5e.10
Chen LC, Ashcroft DM (2007) Risk of myocardial infarction associated with selective COX-2 inhibitors: meta-analysis of randomised controlled trials. Pharmacoepidemiol Drug Saf 16:762–772
Kerney PM, Baigent C, Godwin J, Halls H, Emberson JR, Patrono C (2006) Do selective cyclo-oxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs increase the risk of atherothrombosis? Meta-analysis of randomised trials. BMJ 332:1302–1308
Varas-Lorenzo C, Riera-Guardia N, Calingaert B, Castellsague J, Salvo F, Nicotra F, Sturkenboom M, Perez-Gutthann S (2013) Myocardial infarction and individual nonsteroidal anti-inflammatory drugs meta-analysis of observational studies. Pharmacoepidemiol Drug Saf 22:559–570
Kristensen LE, Jakobsem AK, Askling J, Nilsson F, Jacobsson LTH (2015) Safety of etoricoxib, celecoxib, and nonselective nonsteroidal antiinflammatory drugs in ankylosing spondylitis and other spondyloarthritis patients: a Swedish national population-based cohort study. Arthritis Care Res 67:1137–1149
White WB, West CR, Borer JS, Gorelick PB, Lavange L, Pan SX, Weiner E, Verburg KM (2007) Risk of cardiovascular events in patients receiving celecoxib: a meta-analysis of randomized clinical trials. Am J Cardiol 99:91–98
Chen LC, Ashcroft DM (2006) Do selective COX-2 inhibitors increase the risk of cerebrovascular events? A meta-analysis of randomized controlled trials. J Clin Pharm Ther 31:65–76
Acknowledgements
The authors wish to thank Mr. Joe Perkins for English language editing of the manuscript.
Funding
The present research was conducted by the Dental and Maxillofacial Pathology and Therapeutics research group at the IDIBELL Institute (L’Hospitalet de Llobregat, Spain). The present study did not receive any specific funding from public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors would like to declare the following interests outside the presented work:
Dr. Albert González-Barnadas has participated as a sub-investigator in clinical trials sponsored by Mundipharma (Cambridge, UK).
Dr. Octavi Camps-Font has participated as a sub-investigator in clinical trials sponsored by Mundipharma (Cambridge, UK) and Menarini Richerche (Florence, Italy).
Dr. Pablo Martín-Fatas declares no conflict of interests.
Dr. Rui Figueiredo reports grants, personal fees, and non-financial support from MozoGrau (Valladolid, Spain); personal fees from BioHorizons Ibérica (Madrid, Spain), Inibsa Dental (Lliça de Vall, Spain), Dentsply implants Iberia (Barcelona, Spain), and Araguaney Dental (Barcelona, Spain); and non-financial aid from ADIN Implants (Afula, Israel), and Avinent (Santpedor, Spain) outside the submitted work. Dr. Figueiredo has also participated as a principal investigator in a randomized clinical trial sponsored by Mundipharma (Cambridge, UK) and in another clinical trial as a sub-investigator for Menarini Richerche (Florence, Italy).
Dr. Cosme Gay-Escoda has participated as a principal investigator in several randomized clinical trials sponsored by Mundipharma (Cambridge, UK) and Menarini Richerche (Florence, Italy).
Dr. Eduard Valmaseda-Castellón reports personal fees and non-financial support from MozoGrau (Valladolid, Spain); personal fees from BioHorizons Ibérica (Madrid, Spain), Inibsa Dental (Lliça de Vall, Spain), and Dentsply implants Iberia (Barcelona, Spain); and non-financial aid from Avinent (Santpedor, Spain) outside the submitted work. In addition, Dr. Valmaseda-Castellón has also participated as a sub-investigator in a randomized clinical trial sponsored by Mundipharma (Cambridge, UK).
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
González-Barnadas, A., Camps-Font, O., Martín-Fatás, P. et al. Efficacy and safety of selective COX-2 inhibitors for pain management after third molar removal: a meta-analysis of randomized clinical trials. Clin Oral Invest 24, 79–96 (2020). https://doi.org/10.1007/s00784-019-02910-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-02910-3