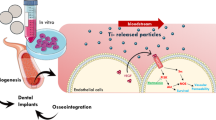
Abstract
Objectives
Endothelial cells play an important role in peri-implant angiogenesis during early bone formation. Therefore, interactions between endothelial progenitor cells (EPCs) and titanium dental implant surfaces are of crucial interest. The aim of our in vitro study was to investigate the reactions of EPCs in contact with different commercially available implant surfaces.
Materials and methods
EPCs from buffy coats were isolated by Ficoll density gradient separation. After cell differentiation, EPC were cultured for a period of 7 days on different titanium surfaces. The test surfaces varied in roughness and hydrophilicity: acid-etched (A), sand-blasted-blasted and acid-etched (SLA), hydrophilic A (modA), and hydrophilic SLA (modSLA). Plastic and fibronectin-coated plastic surfaces served as controls. Cell numbers and morphology were analyzed by confocal laser scanning microscopy. Secretion of vascular endothelial growth factor (VEGF)-A was measured by enzyme-linked immunosorbent assay and expressions of iNOS and eNOS were investigated by real-time polymerase chain reaction.
Results
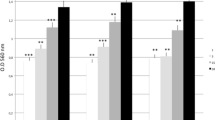
Cell numbers were higher in the control groups compared to the cells of titanium surfaces. Initially, hydrophilic titanium surfaces (modA and modSLA) showed lower cell numbers than hydrophobic surfaces (A and SLA). After 7 days smoother surfaces (A and modA) showed increased cell numbers compared to rougher surfaces (SLA and modSLA). Cell morphology of A, modA, and control surfaces was characterized by a multitude of pseudopodia and planar cell soma architecture. SLA and modSLA promoted small and plump cell soma with little quantity of pseudopodia. The lowest VEGF level was measured on A, the highest on modSLA. The highest eNOS and iNOS expressions were found on modA surfaces.
Conclusions
The results of this study demonstrate that biological behaviors of EPCs can be influenced by different surfaces. The modSLA surface promotes an undifferentiated phenotype of EPCs that has the ability to secrete growth factors in great quantities.
Clinical relevance
In correlation with recent clinical studies these results underline the hypothesis that EPC could promote and increase neovascularization by secreting paracrine factors which support osseointegration of dental implants.





Similar content being viewed by others
References
Buser D, Broggini N, Wieland M, Schenk RK, Denzer AJ, Cochran DL et al (2004) Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 83:529–533
Oates TW, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J et al (2007) Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. Int J Oral Maxillofac Implants 22:755–760
Schätzle M, Männchen R, Balbach U, Hämmerle CH, Toutenburg H, Jung RE (2009) Stability change of chemically modified sandblasted/acid-etched titanium palatal implants. A randomized-controlled clinical trial. Clin Oral Implants Res 20:489–495
Klein MO, Bijelic A, Toyoshima T, Götz H, von Koppenfels RL, Al-Nawas B et al (2010) Longterm response of osteogenic cells on micron and submicron-scale-structured hydrophilic titanium surfaces: sequence of cell proliferation and cell differentiation. Clin Oral Implants Res 21:642–649
Rausch-Fan X, Qu Z, Wieland M, Matejka M, Schedle A (2008) Differentiation and cytokine synthesis of human alveolar osteoblasts compared to osteoblast-like cells (MG63) in response to titanium surfaces. Dent Mater 24:102–110
Zhao G, Schwartz Z, Wieland M, Rupp F, Geis-Gerstorfer J, Cochran DL et al (2005) High surface energy enhances cell response to titanium substrate microstructure. J Biomed Mater Res 74:49–58
Qu Z, Rausch-Fan X, Wieland M, Matejka M, Schedle A (2007) The initial attachment and subsequent behavior regulation of osteoblasts by dental implant surface modification. J Biomed Mater Res 82:658–668
Schliephake H, Scharnweber D, Dard M, Sewing A, Aref A, Roessler S (2005) Functionalization of dental implant surfaces using adhesion molecules. J Biomed Mater Res B Appl Biomater 73:88–96
Kämmerer PW, Heller M, Brieger J, Klein MO, Al-Nawas B, Gabriel M (2011) Immobilisation of linear and cyclic RGD-peptides on titanium surfaces and their impact on endothelial cell adhesion and proliferation. Eur Cell Mater 21:364–372
Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H et al (2001) Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation 103:634–637
Ahmadi H, Baharvand H, Ashtiani SK, Soleimani M, Sadeghian H, Ardekani JM et al (2007) Safety analysis and improved cardiac function following local autologous transplantation of CD133(+) enriched bone marrow cells after myocardial infarction. Curr Neurovasc Res 4:153–160
Ott I, Keller U, Knoedler M, Götze KS, Doss K, Fischer P et al (2005) Endothelial-like cells expanded from CD34+ blood cells improve left ventricular function after experimental myocardial infarction. FASEB J 19:992–994
Krenning G, van Luyn MJ, Harmsen MC (2009) Endothelial progenitor cell-based neovascularization: implications for therapy. Trends Mol Med 15:180–189
Dome B, Dobos J, Tovari J, Paku S, Kovacs G, Ostoros G et al (2008) Circulating bone marrow-derived endothelial progenitor cells: characterization, mobilization, and therapeutic considerations in malignant disease. Cytometry Part A 73:186–193
Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F et al (2008) Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res 103:1327–1334
Yamahara K, Itoh H (2009) Potential use of endothelial progenitor cells for regeneration of the vasculature. Ther Adv Cardiovasc Dis 3:17–27
Risau W (1997) Mechanisms of angiogenesis. Nature 386:671–674
Urbich C, Dimmeler S (2004) Endothelial progenitor cells: characterization and role in vascular biology. Circ Res 95:343–353
Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM et al (2005) Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 39:733–742
Krenning G, van der Strate BW, Schipper M, van Seijen XJ, Fernandes BC, van Luyn MJ et al (2009) CD34(+) cells augment endothelial cell differentiation of CD14(+) endothelial progenitor cells in vitro. J Cell Mol Med 13:2521–2533
Mukai N, Akahori T, Komaki M, Li Q, Kanayasu-Toyoda T, Ishii-Watabe A et al (2008) A comparison of the tube forming potentials of early and late endothelial progenitor cells. Exp Cell Res 314:430–440
Timmermans F, Plum J, Yöder MC, Ingram DA, Vandekerckhove B, Case J (2009) Endothelial progenitor cells: identity defined? J Cell Mol Med 13:87–102
Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG et al (2003) Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res 93:1023–1025
Yang Z, Di Santo S, Kalka C (2010) Current developments in the use of stem cell for therapeutic neovascularisation: is the future therapy “cell-free”? Swiss Med Wkly 140:w13130
Nagano M, Yamashita T, Hamada H, Ohneda K, Kimura K, Nakagawa T et al (2007) Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood 110:151–160
Dimmeler S, Zeiher A (2005) Stammzelltherapie in der kardiologie: stand und perspektiven. Uni-Med, Bremen
Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S (2003) Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9:1370–1376
Guthrie SM, Curtis LM, Mames RN, Simon GG, Grant MB, Scott EW (2005) The nitric oxide pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood 105:1916–1922
Rupp F, Scheideler L, Olshanska N, de Wild M, Wieland M, Geis-Gerstorfer J (2006) Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res 76:323–334
Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H et al (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:E1–E7
Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD (2010) Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials 31:4909–4917
Uggeri J, Guizzardi S, Scandroglio R, Gatti R (2010) Adhesion of human osteoblasts to titanium: a morpho-functional analysis with confocal microscopy. Micron 41:210–219
Kokubu E, Hamilton DW, Inoue T, Brunette DM (2009) Modulation of human gingival fibroblast adhesion, morphology, tyrosine phosphorylation, and ERK 1/2 localization on polished, grooved and SLA substratum topographies. J Biomed Mater Res 91:663–670
Lavenus S, Pilet P, Guicheux J, Weiss P, Louarn G, Layrolle P (2011) Behaviour of mesenchymal stem cells, fibroblasts and osteoblasts on smooth surfaces. Acta Biomaterialia 7:1525–1534
An N, Schedle A, Wieland M, Andrukhov O, Matejka M, Rausch-Fan X (2010) Proliferation, behavior, and cytokine gene expression of human umbilical vascular endothelial cells in response to different titanium surfaces. J Biomed Mater Res 93:364–372
Kawahara H, Soeda Y, Niwa K, Takahashi M, Kawahara D, Araki N (2004) In vitro study on bone formation and surface topography from the standpoint of biomechanics. J Mater Sci Mater Med 15:1297–1307
Schwartz Z, Nasazky E, Boyan BD (2005) Surface microtopography regulates osteointegration: the role of implant surface microtopography in osteointegration. The Alpha Omegan 98:9–19
Wieland M, Textor M, Chehroudi B, Brunette DM (2005) Synergistic interaction of topographic features in the production of bone-like nodules on Ti surfaces by rat osteoblasts. Biomaterials 26:1119–1130
Zhao G, Zinger O, Schwartz Z, Wieland M, Landolt D, Boyan BD (2006) Osteoblast-like cells are sensitive to submicron-scale surface structure. Clin Oral Implants Res 17:258–264
Boyan BD, Schwartz Z, Lohmann CH, Sylvia VL, Cochran DL, Dean DD et al (2003) Pretreatment of bone with osteoclasts affects phenotypic expression of osteoblast-like cells. J Orthop Res 21:638–647
Bornstein MM, Valderrama P, Jones AA, Wilson TG, Seibl R, Cochran DL (2008) Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: a histomorphometric study in canine mandibles. Clin Oral Implants Res 19:233–241
Lai HC, Zhuang LF, Zhang ZY, Wieland M, Liu X (2009) Bone apposition around two different sandblasted, large-grit and acid-etched implant surfaces at sites with coronal circumferential defects: an experimental study in dogs. Clin Oral Implants Res 20:247–253
Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S et al (1994) Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Investig 93:662–670
Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, Iwaguro H et al (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J 18:3964–3972
Brandes RP (2006) Novel faces to old friends: a central role of inducible NO synthase for progenitor cell recruitment and a new antiinflammatory mechanisms of statins. Circ Res 98:303–305
Mayr U, Zou Y, Zhang Z, Dietrich H, Hu Y, Xu Q (2006) Accelerated arteriosclerosis of vein grafts in inducible NO synthase(−/−) mice is related to decreased endothelial progenitor cell repair. Circ Res 98:412–420
Grellier M, Ferreira-Tojais N, Bourget C, Bareille R, Guillemot F, Amede J (2009) Role of vascular endothelial growth factor in the communication between human osteoprogenitors and endothelial cells. J Cell Biochem 106:390–398
Zhang Y, Andrukhov O, Berner S, Matejka M, Wieland M, Rausch-Fan X et al (2010) Osteogenic properties of hydrophilic and hydrophobic titanium surfaces evaluated with osteoblast- like cells (MG63) in coculture with human umbilical vein endothelial cells (HUVEC). Dent Mater 26:1043–1051
Malaval L, Liu F, Roche P, Aubin JE (1999) Kinetics of osteoprogenitor proliferation and osteoblast differentiation in vitro. J Cell Biochem 74:616–627
Asahara T (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275:964–967
Harraz M, Jiao C, Hanlon HD, Hartley RS, Schatteman GC (2001) CD34- blood-derived human endothelial cell progenitors. Stem Cells 19:304–312
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK et al (2004) Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol 24:288–293
Abou-Saleh H, Yacoub D, Theoret JF, Gillis MA, Neagoe PE, Labarthe B et al (2009) Endothelial progenitor cells bind and inhibit platelet function and thrombus formation. Circulation 120:2230–2239
Muscari C, Gamberini C, Carboni M, Basile I, Farruggia G, Bonafè F et al (2007) Different expression of NOS isoforms in early endothelial progenitor cells derived from peripheral and cord blood. J Cell Biochem 102:992–1001
Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A et al (2004) Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res 94:230–238
Li Calzi S, Neu MB, Shaw LC, Kielczewski JL, Moldovan NI, Grant MB (2010) EPCs and pathological angiogenesis: when good cells go bad. Microvasc Res 79:207–216
Acknowledgments
We thank Kathy Taylor for orthographic correction of the article.
Conflict of interest statement
This study was supported by the ITI Foundation for the Promotion of Implantology, Basel, Switzerland, 08/2008 (Dr. Thomas Ziebart).
Author information
Authors and Affiliations
Corresponding author
Additional information
Thomas Ziebart and Anne Schnell contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ziebart, T., Schnell, A., Walter, C. et al. Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces. Clin Oral Invest 17, 301–309 (2013). https://doi.org/10.1007/s00784-012-0691-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-012-0691-7