Abstract
Metabolism of food protein by gut microbes produce trimethylamine which on oxidation by hepatic flavin-containing monooxygenases is transformed to trimethylamine-N-oxide (TMAO). TMAO has recently been implicated as a biomarker for atherosclerosis. TMAO, as (CH3)3N+–O−), is ionic and so a hydrophilic molecule that is freely available in blood plasma. For the effective interaction with lipid-soluble molecules, TMAO should be phase transferred to the lipid site. We show that the free TMAO is effectively bonded to zinc protoporphyrin IX dimethyl ester [ZnPPDME] to yield [TMAOZnPPDME] using phase transfer reaction. The zinc protoporphyrin IX, [ZnPP], in general, available in blood may form [TMAOZnPP] complex. The nature of such interaction between TMAO and [ZnPP] has been structurally shown using a model complex, [TMAOZnTPP] (TPP = tetraphenylporphyrin). These complexes readily move from the polar plasma to the non-polar (lipid) site to act as the oxo-transfer agent to oxidize cholesterol causing atherosclerosis. Chromatographic and circular dichroism (CD) studies show that either TMAO or [ZnPP] alone cannot oxidize cholesterol.
Graphic abstract
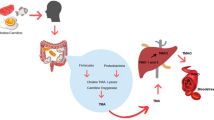
Free TMAO bonded with zinc-protoporphyrin IX, [ZnPP], in blood plasma as [TMAOZnPP] is transported to the lipid site and this is the reacting species to oxidize cholesterol causing atherosclerosis.






Similar content being viewed by others
References
Wang Z, Klipfell E, Bennett BJ et al (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472:57–63
Tang WHW, Wang Z, Levison BS et al (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 368:1575–1584
Cho CE, Caudill MA (2017) Trimethylamine-N-oxide: friend, foe, or simply caught in the cross-fire? Trends Endrocrinol Metab 28:121–130
Luc G, Fruchart JC (1991) Oxidation of lipoproteins and atherosclerosis. Am J Clin Nutr 53:206S-209S
Witztum JL, Steinberg D (1991) Role of oxidized low density lipoprotein in atherogenesis. J Clin Investig 88:1785–1792
Jialal I, Devaraj S (1996) Low-density lipoprotein oxidation, antioxidants, and atherosclerosis: a clinical biochemistry perspective. Clin Chem 42:498–506
Libby P (2002) Inflammation in atherosclerosis. Nature 420:868–874
Ross R, Glomset JA (1976) The pathogenesis of atherosclerosis. N Engl J Med 295:369–377
Jonasson L, Holm J, Skalli O et al (1986) Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis 6:131–138
Hansson GK (2005) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Lusis AJ (2000) Atherosclerosis. Nature 407:233–241
Libby P (2000) Changing concepts of atherogenesis. J Intern Med 247:349–358
Bäckhed F (2013) Meat-metabolizing bacteria in atherosclerosis. Nat Med 19:533–534
Koeth RA, Wang Z, Levison BS et al (2013) Intestinal microbiota metabolism of l-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 19:576–585
Cashman JR, Zhang J (2006) Human flavin-containing monooxygenases. Annu Rev Pharmacol Toxicol 46:65–100
Cho CE, Taesuwan S, Malysheva OV et al (2017) Trimethylamine-N-oxide (TMAO) response to animal source foods varies among healthy young men and is influenced by their gut microbiota composition: a randomized controlled trial. Mol Nutr Food Res 61:1770016
Bordoni L, Sawicka AK et al (2020) A pilot study on the effects of l-carnitine and trimethylamine-N-oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women. Int J Mol Sci 21:1047
Papandreou C, Moré M, Bellamine A (2020) Trimethylamine N-oxide in relation to cardiometabolic health—cause or effect? Nutrients 12:1330
Velasquez MT, Ramezani A, Manal A et al (2016) Trimethylamine N-oxide: the good, the bad and the unknown. Toxins (Basel) 8:326
Subramaniam S, Fletcher C (2018) Trimethylamine N-oxide: breathe new life. Br J Pharmacol 175:1344–1353
Jaworska K, Hering D et al (2019) TMA, a forgotten uremic toxin, but not TMAO, is involved in cardiovascular pathology. Toxins 11:490
Bordoni L, Samulak JJ et al (2020) Trimethylamine N-oxide and the reverse cholesterol transport in cardiovascular disease: a cross-sectional study. Sci Rep 10:18675
Bordoni L, Fedeli D, Piangerelli M et al (2020) Gender-related differences in trimethylamine and oxidative blood biomarkers in cardiovascular disease patients. Biomedicines 8:238
Poli G, Sottero B, Gargiulo S et al (2009) Cholesterol oxidation products in the vascular remodeling due to atherosclerosis. Mol Aspects Med 30:180–189
Javitt NB (1994) Bile acid synthesis from cholesterol: regulatory and auxiliary pathways. FASEB J 8:1308–1311
Labbe RF, Vreman HJ, Stevenson DK (1999) Zinc protoporphyrin: a metabolite with a mission. Clin Chem 45:2060–2072
Labbé RF (1992) Clinical utility of zinc protoporphyrin. Clin Chem 38:2167–2168
Lamola AA, Eisinger J, Blumberg WE (1980) Erythrocyte protoporphyrin/heme ratio by hematofluorometry. Clin Chem 26:677–678
Cheng C, Noordeloos AM, Jeney V et al (2009) Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation 119:3017–3027
Sevanian A, McLeod LL (1987) Cholesterol autoxidation in phospholipid membrane bilayers. Lipids 22:627–636
Smith LL, Johnson BH (1989) Biological activities of oxysterols. Free Radic Biol Med 7:285–332
Guardiola F, Codony R, Addis PB et al (1996) Biological effects of oxysterols: current status. Food Chem Toxicol 34:193–211
Russell DW (2000) Oxysterol biosynthetic enzymes. Biochim Biophys Acta 1529:126–135
Sottero B, Gamba P, Gargiulo S et al (2009) Cholesterol oxidation products and disease: an emerging topic of interest in medicinal chemistry. Curr Med Chem 16:685–705
Bitman J, Wood DL (1982) J Liq Chromatogr 5:1155–1162
Lebovics VK (2002) In cholesterol and phytosterol oxidation products. AOCS Publishing, pp 105–117
Kumar A, Maji S, Dubey P, Abhilash G, Pandey S, Sarkar S (2007) Tetrahedron Lett 48:7287
Fuhrhop JH, Smith KM (1975) In porphyrins and metalloporphyrins (edited by K. M. Smith). Elsevier, Amsterdam, p 798
Sarkar R (2012) PhD Thesis. Dept. of Chemistry, IIT Kanpur, p 60
Berto TC, Praneeth VKK, Goodrich LE et al (2009) Iron-Porphyrin NO complexes with covalently attached N-donor ligands: formation of a stable six-coordinate species in solution. J Am Chem Soc 131:17116–17126
Lebovics VK (2002) Determination of cholesterol oxidation products by thin-layer chromatography. AOCS Publishing, pp 105–117
Moula G, Bose M, Sarkar S (2013) Replica of a fishy enzyme: structure-function analogue of trimethylamine-N-oxide reductase. Inorg Chem 52:5316–5327
Tanekazu K, Hiroshi M (1962) Polarography of pyridine N-oxide and its alkyl derivatives. Bull Chem Soc Jpn 35:1549–1551
Ochiai E (1967) Aromatic amine oxides. Elsevier, Amsterdam, pp 6–17 (91–97)
Simala-Grant JL, Weiner JH (1996) Kinetic analysis and substrate specificity of Escherichia coli dimethyl sulfoxide reductase. Microbiology 142:3231–3239
Acknowledgements
This research was funded by SERB-DST (EMR/2015/001328), New. Delhi, to SS. NP is thankful to CSIR (08/003/0108(2015)-EMR-I), New Delhi for a SRF and RS is thankful to UGC, New Delhi, for granting him a DS Kothari Post-Doctoral Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paul, N., Sarkar, R. & Sarkar, S. Zinc protoporphyrin–trimethylamine-N-oxide complex involves cholesterol oxidation causing atherosclerosis. J Biol Inorg Chem 26, 367–374 (2021). https://doi.org/10.1007/s00775-021-01861-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-021-01861-z