Abstract
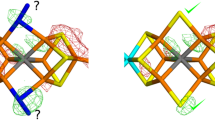
Partial symmetry, i.e., the presence of more than one molecule in the asymmetric unit of a crystal, is a relatively rare phenomenon in small-molecule crystallography, but is quite common in protein crystallography, where it is typically known as non-crystallographic symmetry (NCS). Several papers in literature propose molecular determinants such as crystal contacts, thermal factors, or TLS parameters as an explanation for the phenomenon of intrinsic asymmetry among molecules that are in principle equivalent. Nevertheless, are all of the above determinants the cause or are they rather the effect? In the general frame of the NCS often observed in crystals of biomolecules, this paper deals with nickel(II)-substituted human carbonic anhydrase(II) (hCAII) and its SAD structure determination at the nickel edge. The structure revealed two non-equivalent molecules in the asymmetric unit, the presence of a secondary nickel-binding site at the N-terminus of both molecules (which had never been found before in the nickel-substituted enzyme) and two different coordination geometries of the active site nickel (hexa-coordinated in one molecule and mainly penta-coordinated in the other). The above-mentioned standard molecular crystallographic determinants of this asymmetry are analyzed and presented in detail for this particular case. From these considerations, we speculate on the existence of a fundamental, although yet unknown, common cause for the partial symmetry that is so often encountered in X-ray structures of biomolecules.









Similar content being viewed by others
Notes
The simple counting of the water molecules present in the pdb files of both structures does not seem to be a reliable parameter in the evaluation of the dehydration. In fact, the much higher resolution of our structure (1.4 Å vs 1.9 Å) greatly increases the number of water molecule characterized by a clear electron density, making this comparison devoid of significance.
References
Fichtner K (1986) Comput Math Appl-B 12:751–762
Tripp BC, Smith K, Ferry JG (2001) J Biol Chem 276:48615–48618
Lipton AS, Heck RW, Ellis PD (2004) J Am Chem Soc 126:4735–4739
Bertini I, Canti G, Luchinat C, Borghi E (1983) J Inorg Biochem 18:221–229
Khalifah RG (1973) Proc Natl Acad Sci USA 70:1986–1989
Lindskog S (1997) Pharmacol Ther 74:1–20
Sethi KK, Vullo D, Verma SM, Tanc M, Carta F, Supuran CT (2013) Bioorg Med Chem 21:5973–5982
Cerofolini L, Giuntini S, Louka A, Ravera E, Fragai M, Luchinat C (2017) J Phys Chem B 121:8094–8101
Hakansson K, Wehnert A, Liljas A (1994) Acta Crystallogr D Biol Crystallogr 50:93–100
Coleman JE (1967) J Biol Chem 242:5212–5219
Avvaru BS, Arenas DJ, Tu C, Tanner DB, McKenna R, Silverman DN (2010) Arch Biochem Biophys 502:53–59
Bertini I, Luchinat C (1984) Ann N Y Acad Sci 429:89–98
Bertini I, Luchinat C, Scozzafava A (1982) Struct Bonding 48:45–92
Bertini I, Canti G, Luchinat C, Scozzafava A (1978) J Am Chem Soc 100:4873–4877
Cerofolini L, Staderini T, Giuntini S, Ravera E, Fragai M, Parigi G, Pierattelli R, Luchinat C (2018) J Biol Inorg Chem 23:71–80
Cox JD, Hunt JA, Compher KM, Fierke CA, Christianson DW (2000) Biochemistry 39:13687–13694
Kabsch W (2010) Acta Crystallogr D Biol Crystallogr 66:125–132
Vonrhein C, Blanc E, Roversi P, Bricogne G (2007) Methods Mol Biol 364:215–230
Cowtan K (2006) Acta Cryst D 62:1002–1011
Langer G, Cohen SX, Lamzin VS, Perrakis A (2008) Nat Protoc 3:1171–1179
Murshudov GN, Skubàk P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA (2011) Acta Crystallogr D Biol Crystallogr 67:355–367
Emsley P, Cowtan K (2004) Acta Crystallogr D Biol Crystallogr 60:2126–2132
Chen VB, Arendall WB III, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) Acta Crystallogr D Biol Crystallogr 66:12–21
Krissinel E, Henrick K (2007) J Mol Biol 372:774–797
Håkansson K, Carlsson M, Svensson LA, Liljas A (1992) J Mol Biol 227:1192–1204
Bertarello A, Schubeis T, Fuccio C, Ravera E, Fragai M, Parigi G, Emsley L, Pintacuda G, Luchinat C (2017) Inorg Chem 56:6624–6629
Bertini I, Borghi E, Luchinat C (1978) Bioinorg Chem 9:495–504
Robbins AH, Domsic JF, Agbandje-McKenna M, McKenna R (2010) Acta Crystallogr D Biol Crystallogr 66:950–952
Vellieux FMD, Dijkstra BW (1997) J Appl Crystallogr 30:396–399
Acknowledgements
The experiments were performed on beamline ID23-1 at the European Synchrotron Radiation Facility (ESRF), Grenoble, France. We are grateful to Local Contact at the ESRF for providing assistance in using beamline ID23-1. The authors acknowledge the support and the use of resources of Instruct-ERIC, a Landmark ESFRI project, and specifically the CERM/CIRMMP Italy Centre. This work has been supported by Fondazione Cassa di Risparmio di Firenze, the European Commission (contract #675858). J.P.S. acknowledges FCT for the doctoral fellowship PD/BD/135180/2017 integrated in the Ph.D. Program in NMR applied to chemistry, materials, and biosciences.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Silva, J.M., Giuntini, S., Cerofolini, L. et al. Non-crystallographic symmetry in proteins: Jahn–Teller-like and Butterfly-like effects?. J Biol Inorg Chem 24, 91–101 (2019). https://doi.org/10.1007/s00775-018-1630-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-018-1630-0