Abstract
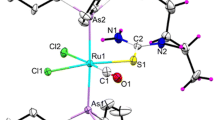
A series of new Ni(II) complexes containing indole-based thiosemicarbazone ligands was synthesized and characterized by elemental analyses, and UV–visible, FT-IR, 1H & 13C NMR and mass spectroscopic techniques. The Ni(II) complexes (1–4) bear the general formula [Ni{C10H9N2NHCSNH(R)}2] where R = hydrogen (1), 4-methyl (2), 4-phenyl (3) and 4-cyclohexyl (4). Molecular structure of ligands (L3 and L4) and complexes (2, 3 and 4) was confirmed by single crystal X-ray crystallography. Four coordinated Ni(II) complexes showed square planar geometry. The interaction of the Ni(II) complexes with calf thymus DNA (CT-DNA) has been evaluated by absorption spectroscopic and ethidium bromide (EB) competitive binding studies, which revealed the intercalative interaction of the complexes with CT-DNA. Gel electrophoresis experiments showed the cleavage of DNA by the complexes without any external agent. Further, the interaction of the complexes with bovine serum albumin (BSA) was investigated using UV–visible, fluorescence and synchronous fluorescence spectroscopic methods, which showed that the complexes could bind strongly with BSA. Molecular docking was employed to understand the binding of the Ni(II) complexes with the molecular target B-DNA, human DNA topoisomerase I and BSA. All the Ni(II) complexes possess high antioxidant activity against 2-2-diphenyl-1-picrylhydrazyl (DPPH) radical and antihaemolytic activity. In addition, in vitro cytotoxicity of the Ni(II) complexes against lung cancer (A549), human breast cancer (MCF7) and mouse embryonic fibroblasts (L929) cell lines was investigated. Complex 4 has high cytotoxicity. The mode of cell death effected by complex 4 has been explored using Hoechst 33258 staining.
Graphical abstract
Nickel(II) complexes of thiosemicarbazone ligands were synthesized and their DNA/protein binding, DNA cleavage and cytotoxicity abilities were studied.











Similar content being viewed by others
References
Hambley TW (2007) Dalton Trans 43:4929–4937
Orvig C, Abrams M (1999) Chem Rev 99:2201–2203
Thompson KH, Orvig C (2006) Dalton Trans 14:761–764
Santini C (2010) Chem Rev 254:1179–1218
Reedijk J (2008) Macromol Symp 270:193–201
Fricker SP (2007) Dalton Trans 43:4903–4917
Beraldo H, Gambino D (2004) Mini Rev Med Chem 4:31–39
Abid M, Agarwal SM, Azam A (2008) Eur J Med Chem 43:2035–2039
Mahalingam V, Chitrapriya N, Fronczek FR, Natarajan K (2008) Polyhedron 27:2743–2750
Liu MC, Lin TS, Sartorelli AC (1992) J Med Chem 35:3672–3677
Dilovic I, Rubcic M, Vrdoljak V, Pavelic SK, Kralj M, Piantanida Cindric IM (2008) Bioorg Med Chem 16:5189–5198
Duffy KJ, Shaw AN, Delorme E, Dillon SB, Miller CE, Giampa L, Huang Y, Keenan RM, Lamb P, Liu N, Miller SG, Price AT, Rosen J, Smith H, Wiggall KJ, Zhang L, Luengo JI (2002) J Med Chem 45:3573–3575
Cowley AR, Dilworth JR, Donnelly PS, Labisbal E, Sousa A (2002) J Am Chem Soc 124:5270–5271
Prabhakaran R, Kalaivani P, Jayakumar R, Zeller M, Hunter AD, Renukadevi SV, Ramachandran E, Natarajan K (2011) Metallomics 3:42–48
Kalaivani P, Prabhakaran R, Dallemer F, Poornima P, Vaishnavi E, Ramachandran E, Padma VV, Renganathan R, Natarajan K (2012) Metallomics 4:101–113
Ramachandran E, Kalaivani P, Prabhakaran R, Rath NP, Brinda S, Poornima P, Padma VV, Natarajan K (2012) Metallomics 4:218–227
Kalaivani P, Prabhakaran R, Ramachandran E, Dallemer F, Paramaguru G, Renganathan R, Poornima P, Padma VV, Natarajan K (2012) Dalton Trans 4:2486–2499
Senthil Raja D, Bhuvanesh NSP, Natarajan K (2011) Inorg Chem 50:12852–12866
Oliveira RBD, Fagundes EMDS, Soares RPP, Andrade AA, Krettli AU, Zani CL (2008) Eur J Med Chem 43:1983–1988
Walcourt A, Loyevsky M, Lovejoy DB, Gordeuk VR, Richardson DR (2004) Int J Biochem Cell Biol 36:401–407
Ramachandran E, Senthil Raja D, Mike JL, Wagner TR, Zeller M, Natarajan K (2012) RSC Adv 2:8515–8525
Sundberg RJ (1996) Indoles. Academic Press, London
Bandini M, Eichholzer A (2009) Angew Chem Int Ed 48:9608–9644
Gillman PK, Bartlett JR, Bridges PK, Hunt A, Patel AJ, Kantamaneni BD, Curzon G (1981) J Neurochem 37:410–417
Higdon JV, Delage B, Williams DE, Dashwood RH (2007) Pharmacol Res 55:224–236
Yan XJ, Qi M, Telusma G, Yancopoulos S, Madaio M, Satoh M, Reeves WH, Teichberg S, Kohn N, Auborn K, Chiorazzi N (2009) Clin Immunol 131:481–494
Park N, Kim JK, Park WT, Cho JW, Lim YP, Park SU (2011) Mol Biol Rep 38:4947–4953
Chang X, Tou JC, Hong C, Kim HA, Riby JE, Firestone GL, Bjeldanes LF (2005) Carcinogenesis 26:771–778
Cope RB, Loehr C, Dashwood R, Kerkvliet NI (2006) Photochem Photobiol Sci 5:499–507
Dahlöf C (2005) Therapy 2:349–356
Behari J, Zeng G, Otruba W, Thompson M, Muller P, Micsenyi A, Sekhon S, Leoni L, Monga S (2007) J Hepatol 46:849–857
William H, Frishman MDN (1983) Engl J Med 308:940–945
APEX2 Program for data collection and integration on area detectors. BRUKER AXS Inc., Madison
SADABS, Sheldrick GM Program for absorption correction of area detector frames. BRUKER AXS Inc., Madison
Sheldrick GM (2008) Acta Cryst A64:112–122
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) J Appl Cryst 42:339–341
Mitra K, Basu U, Khan I, Maity B, Kondaiah P, Chakravarty AR (2014) Dalton Trans 43:751–763
Simpson JA, Cheeseman KH, Smith SE, Dean RT (1988) Biochem J 54:519–523
Rajput C, Rutkaite R, Swanson L, Haq I, Thomas JA (2006) Chem Eur J 12:4611–4619
Cohen G, Eisenberg H (1969) Biopolymers 8:45–55
Deshpande MS, Kumbhar AA, Kumbhar AS, Kumbhakar M, Pal H, Sonawane UB, Joshi RR (2009) Bioconj Chem 20:447–459
Jeyalakshmi K, Haribabu J, Bhuvanesh NSP, Karvembu R (2016) Dalton Trans 45:12518–12531
Haribabu J, Jeyalakshmi K, Arun Y, Bhuvanesh NSP, Perumal PT, Karvembu R (2015) RSC Adv 5:46031–46049
Selvakumaran N, Bhuvanesh NSP, Karvembu R (2014) Dalton Trans 43:16395–16410
Oktay M, Gulein I, Kufreviolglu I, Labenson-Wiss U (2003) Technol 36:263–271
Haribabu J, Subhashree GR, Saranya S, Gomathi K, Karvembu R, Gayathri D (2015) J Mol Struct 1094:281–291
Yang ZG, Sun HX, Fang WH (2005) Vaccine 23:5196–5203
Mossman T (1983) J Immunol Methods 65:55–63
Stander A, Marais S, Stivaktas V (2009) J Ethnopharmacol 124:45–60
Prabhakaran R, Karvembu R, Hashimoto T, Shimizu K, Natarajan K (2005) Inorg Chim Acta 358:2093–2096
Kalaivani P, Umadevi C, Prabhakaran R, Dallemer F, Mohan PS, Natarajan K (2014) Polyhedron 80:97–105
Vimala G, Govindaraj J, Haribabu J, Karvembu R, Subbiah Pandi A (2014) Acta Cryst E70:o1151
Murrayrust P, Burgi HB, Dunitz JD (1975) J Am Chem Soc 97:921–922
Keinan S, Avnir D (2001) J Chem Soc Dalton Trans 6:941–947
Senthil Raja D, Paramaguru G, Bhuvanesh NSP, Reibenspies JH, Renganathan R, Natarajan K (2011) Dalton Trans 40:4548–4559
Liu ZC, Wang BD, Li B, Wang Q, Yang ZY, Li TR, Li Y (2010) Eur J Med Chem 45:5353–5361
Pyle AM, Rehmann JP, Meshoyrer R, Kumar CV, Turro NJ, Barton JK (1989) J Am Chem Soc 111:3051–3058
Jeyalakshmi K, Selvakumaran N, Bhuvanesh NSP, Sreekanth A, Karvembu R (2014) RSC Adv 4:17179–17195
Lakowicz JR, Webber G (1973) Biochemistry 12:4161–4170
Baguley BC, Lebret M (1984) Biochemistry 23:937–943
Nyarko E, Hanada N, Habib A, Tabata M (2004) Inorg Chim Acta 357:739–745
Lepecq JB, Paoletti C (1967) J Mol Biol 27:87–106
Jeyalakshmi K, Arun Y, Bhuvanesh NSP, Perumal PT, Sreekanth A, Karvembu R (2015) Inorg Chem Front 2:780–798
Kumar CV, Asuncion EH (1993) J Am Chem Soc 115:8547–8553
Ivanov VI, Minchenkova LE, Schyolkina AK, Poletayer AI (1973) Biopolymers 12:89–110
Tamilselvi P, Palaniandavar M (2002) Inorg Chim Acta 337:420–428
Krishnamoorthy P, Sathyadevi P, Butorac RR, Cowley AH, Bhuvanesh NSP, Dharmaraj N (2012) Dalton Trans 41:4423–4436
Goswami TK, Gadadhar S, Balaji B, Gole B, Karande AA, Chakravarty AR (2014) Dalton Trans 43:11988–11999
Liu J, Zheng WJ, Shi S, Tan CP, Chen JC, Zheng KC, Ji LN (2008) J Inorg Biochem 102:193–202
Raja DS, Bhuvanesh NSP, Natarajan K (2011) Eur J Med Chem 46:4584–4594
Ramachandran E, Thomas SP, Poornima P, Kalaivani P, Prabhakaran R, Padma VV, Natarajan K (2012) Eur J Med Chem 50:405–415
Bhattacharyya M, Chaudhuri U, Poddar RK (1990) Biochem Biophys Res Commun 167:1146–1153
Senthil Raja D, Bhuvanesh NSP, Natarajan K (2012) Dalton Trans 41:4365–4377
Sanner MF (1999) J Mol Graphics Mod 17:57–61
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) J Comput Chem 30:2785–2791
Protein Data Bank. http://www.rcsb.org/pdb
Gao E, Sun N, Zhang S, Ding Y, Qiu X, Zhan Y, Zhu M (2016) Eur J Med Chem 121:1–11
Icsel C, Yilmaz VT, Kaya Y, Samli H, Harrison WTA, Buyukgungor O (2015) Dalton Trans 44(15):6880–6895
Xiao XS, Antony S, Pommier Y, Cushman M (2005) J Med Chem 48:3231–3238
Sankareswari VG, Vinod D, Mahalakshmi A, Alamelu M, Kumaresan G, Ramaraj R, Rajagopal S (2014) Dalton Trans 43:3260–3272
Ramachandran E, Senthil Raja D, Bhuvanesh NSP, Natarajan K (2012) Dalton Trans 41:13308–13323
Haribabu J, Subhashree GR, Saranya S, Gomathi K, Karvembu R, Gayathri D (2016) J Mol Struct 1110:185–195
Blagosklonny M, EIdiery WS (1996) Int J Cancer 67:386–392
Dobrova A, Platzer S, Bacher F, Milunovic MNM, Dobrov A, Spengler G, Enyedy ÉA, Novitchid G, Arion VB (2016) Dalton Trans 45:13427–13439
Kalaivani P, Saranya S, Poornima P, Prabhakaran R, Dallemer F, Vijaya Padma V, Natarajan K (2014) Eur J Med Chem 82:584–599
Shawish HB, Paydar M, Looi CY, Wong YL, Movahed E, Abdul Halim SN, Wong WF, Mustafa MR, Jamil Maah M (2014) Transition Met Chem 39:81–94
Mazlan NA, Begum TS, Ravoof A, Edward R, Tiekink T, Mohamed Tahir MI, Veerakumarasivam A, Crouse KA (2014) Transition Met Chem 39:633–639
Komeda S, Lutz M, Spek AL, Yamanaka Y, Sato T, Chikuma M, Reedijk J (2002) J Am Chem Soc 124:4738–4746
Ghobrial IM, Witzig TE, Adjei AA (2005) CA Cancer J Clin 55:178–194
Ramakrishnan S, Shakthipriya D, Suresh E, Periasamy VS, Akbarsha MA, Palaniandavar M (2011) Inorg Chem 50:6458–6471
Busto N, Valladolid J, Martinez-Alonso M, Lozano HJ, Jalon FA, Manzano BR, Rodriguez AM, Carrion MC, Biver T, Leal JM, Espino G, Garcia B (2013) Inorg Chem 52:9962–9974
Caruso F, Monti E, Matthews J, Rossi M, Gariboldi MB, Pettinari C, Pettinari R, Marchetti F (2014) Inorg Chem 53:3668–3677
Acknowledgements
JH thanks the University Grants Commission for the fellowship (F1-17.1/2012-13/RGNF-2012-13-ST-AND-18716).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
775_2016_1424_MOESM1_ESM.pdf
Crystallographic data for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Centre (CCDC) as supplementary publication numbers (CCDC 1451697, CCDC 1451698, CCDC 1451699, CCDC 1451700 and CCDC 1451701 for L3, L4, 2, 3 and 4, respectively). Copies of the data can be obtained free of charge from the CCDC (http://www.ccdc.cam.ac.uk) (PDF 2668 kb)
Rights and permissions
About this article
Cite this article
Haribabu, J., Jeyalakshmi, K., Arun, Y. et al. Synthesis of Ni(II) complexes bearing indole-based thiosemicarbazone ligands for interaction with biomolecules and some biological applications. J Biol Inorg Chem 22, 461–480 (2017). https://doi.org/10.1007/s00775-016-1424-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-016-1424-1