Abstract
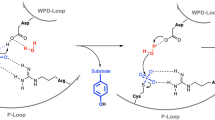
Serine/threonine protein phosphatases have been described in many pathogenic bacteria as essential enzymes involved in phosphorylation-dependent signal transduction pathways and frequently associated with the virulence of these organisms. An inspection of Mycoplasma synoviae genome revealed the presence of a gene (prpC) encoding a putative protein phosphatase of the protein phosphatase 2C (PP2C) subfamily. Here, we report a complete biochemical characterization of M. synoviae phosphatase (PrpC) and the particular role of metal ions in the structure–function relationship of this enzyme. PrpC amino acid sequence analysis revealed that all the residues involved in the dinuclear metal center and the putative third metal ion-coordinating residues, conserved in PP2C phosphatases, are present in PrpC. PrpC is a monomeric protein able to dephosphorylate phospho-substrates with Mn2+ ions’ dependence. Thermal stability analysis demonstrated the enzyme stability at mild temperatures and the influence of Mn2+ ions in this property. Mass spectrometry analysis suggested that three metal ions bind to PrpC, two of which with an apparent high-affinity constant. Mutational analysis of the putative third metal-coordinating residues, Asp122 and Arg164, revealed that these variants exhibited a weaker binding of manganese ions, and that both mutations affected PrpC phosphatase activity. According to these results, PrpC is a metal-dependent protein phosphatase member with an improved stability in the holo form and with Asp122, possibly implicated in the third metal-binding site, essential to catalytic activity.




Similar content being viewed by others
Abbreviations
- ESI-MS:
-
Electrospray ionization-mass spectrometry
- HAD:
-
Haloacid dehalogenase
- MALDI-TOF/TOF:
-
Matrix-assisted laser desorption/ionization-time-of-flight
- Mw:
-
Molecular weight
- FCP/SCP:
-
[TFIIF(transcription initiation factor IIF)-associating component of CTD (C-terminal domain) phosphatase/small CTD phosphatase]
- WT:
-
Wild type
References
Razin S, Yogev D, Naot Y (1998) Microbiol Mol Biol Rev 62:1094–1156
Citti C, Blanchard A (2013) Trends Microbiol 21:196–203
Jeffery N, Gasser RB, Steer PA, Noormohammadi AH (2007) Microbiology 153:2679–2688
Dusanic D, Bencina D, Oven I, Cizelj I, Bencina M, Narat M (2012) Vet Res 43:7
May M, Brown DR (2009) J Bacteriol 191:3588–3593
Koul A, Choidas A, Treder M, Tyagi AK, Drlica K, Singh Y, Ullrich A (2000) J Bacteriol 182:5425–5432
Schmidl SR, Gronau K, Pietack N, Hecker M, Becher D, Stülke J (2010) Mol Cell Proteomics 9:1228–1242
Vasconcelos ATR, Ferreira HB, Bizarro CV, Bonatto SL, Carvalho MO, Pinto PM, Almeida DF, Almeida LGP, Almeida R, Alves-Filho L, Assunção EN, Azevedo VAC, Bogo MR, Brigido MM, Brocchi M, Burity HA, Camargo AA, Camargo SS, Carepo MS, Carraro DM, de Mattos Cascardo JC, Castro LA, Cavalcanti G, Chemale G, Collevatti RG, Cunha CW, Dallagiovanna B, Dambrós BP, Dellagostin OA, Falcão C, Fantinatti-Garboggini F, Felipe MSS, Fiorentin L, Franco GR, Freitas NSA, Frías D, Grangeiro TB, Grisard EC, Guimarães CT, Hungria M, Jardim SN, Krieger MA, Laurino JP, Lima LFA, Lopes MI, Loreto ÉLS, Madeira HMF, Manfio GP, Maranhão AQ, Martinkovics CT, Medeiros SRB, Moreira MAM, Neiva M, Ramalho-Neto CE, Nicolás MF, Oliveira SC, Paixão RFC, Pedrosa FO, Pena SDJ, Pereira M, Pereira-Ferrari L, Piffer I, Pinto LS, Potrich DP, Salim ACM, Santos FR, Schmitt R, Schneider MPC, Schrank A, Schrank IS, Schuck AF, Seuanez HN, Silva DW, Silva R, Silva SC, Soares CMA, Souza KRL, Souza RC, Staats CC, Steffens MBR, Teixeira SMR, Urmenyi TP, Vainstein MH, Zuccherato LW, Simpson AJG, Zaha A (2005) J Bacteriol 187:5568–5577
Shi Y (2009) Cell 139:468–484
Moorhead GBG, De wever V, Templeton G, Kerk D (2009) Biochem J 417:401–409
Brautigan DL (2013) FEBS J 280:324–325
Das AK, Helps NR, Cohen PT, Barford D (1996) EMBO J 15:6798–6809
Wehenkel A, Bellinzoni M, Schaeffer F, Villarino A, Alzari PM (2007) J Mol Biol 374:890–898
Schlicker C, Fokina O, Kloft N, Grüne T, Becker S, Sheldrick GM, Forchhammer K (2008) J Mol Biol 376:570–581
Pullen KE, Ng H-L, Sung P-Y, Good MC, Smith SM, Alber T (2004) Structure 12:1947–1954
Bellinzoni M, Wehenkel A, Shepard W, Alzari PM (2007) Structure 15:863–872
Rantanen MK, Lehtiö L, Rajagopal L, Rubens CE, Goldman A (2007) FEBS J 274:3128–3137
Tanoue K, Miller Jenkins LM, Durell SR, Debnath S, Sakai H, Tagad HD, Ishida K, Appella E, Mazur SJ (2013) Biochemistry 52:5830–5843
Su J, Forchhammer K (2013) FEBS J 280:694–707
Altschul SF, Wootton JC, Gertz EM, Agarwala R, Morgulis A, Schäffer AA, Yu YK (2005) FEBS J 272:5101–5109
Thompson JD, Higgins DG, Gibson TJ (1994) Nucleic Acids Res 22:4673–4680
Arnold K, Bordoli L, Kopp J, Schwede T (2006) Bioinformatics 22:195–201
Hasegawa H, Holm L (2009) Curr Opin Struct Biol 19:341–348
Krissinel E, Henrick K (2004) Acta Crystallogr Sect D Biol Crystallogr 60:2256–2268
DeLano WL (2002) The pymol molecular graphics system. DeLano Scientific, San Carlos
Menegatti ACO, Tavares CP, Vernal J, Klein CS, Huergo L, Terenzi H (2010) Vet Microbiol 145:134–141
Fathi A-R, Krautheim A, Lucke S, Becker K, Juergen Steinfelder H (2002) Anal Biochem 310:208–214
Whitmore L, Wallace BA (2008) Biopolymers 89:392–400
Obuchowski M, Madec E, Delattre D, Boël G, Iwanicki A, Foulger D, Séror SJ (2000) J Bacteriol 182:5634–5638
Bork P, Brown NP, Hegyi H, Schultz J (1996) Protein Sci 5:1421–1425
Ariño J, Casamayor A, González A (2011) Eukaryot Cell 10:21–33
Fjeld CC, Denu JM (1999) J Biol Chem 274:20336–20343
Halbedel S, Busse J, Schmidl SR, Stülke J (2006) J Biol Chem 281:26253–26259
Su J, Schlicker C, Forchhammer K (2011) J Biol Chem 286:13481–13488
Lai SM, Moual HL (2005) Microbiology 151:1159–1167
Bodart J-F, Béchard D, Bertout M, Rousseau A, Gannon J, Vilain J-P, Flament S (1999) FEBS Lett 457:175–178
Matiollo C, Ecco G, Menegatti ACO, Razzera G, Vernal J, Terenzi H (2013) Biochim Biophys Acta 1834:191–196
Chi M-C, Wu T-J, Chuang T-T, Chen H-L, Lo H-F, Lin L-L (2010) Protein J 29:572–582
Geoghegan KF, Dixon HBF, Rosner PJ, Hoth LR, Lanzetti AJ, Borzilleri KA, Marr ES, Pezzullo LH, Martin LB, LeMotte PK, McColl AS, Kamath AV, Stroh JG (1999) Anal Biochem 267:169–184
Perozzo R, Folkers G, Scapozza L (2004) J Recept Signal Transduct 24:1–52
Burnside K, Rajagopal L (2011) Future Microbiol 6:747–761
Agarwal S, Agarwal S, Jin H, Pancholi P, Pancholi V (2012) J Biol Chem 287:9147–9167
Zhang W, Shi L (2004) Microbiology 150:4189–4197
Dupeux F, Antoni R, Betz K, Santiago J, Gonzalez-Guzman M, Rodriguez L, Rubio S, Park S-Y, Cutler SR, Rodriguez PL, Márquez JA (2011) Plant Physiol 156:106–116
Melcher K, Ng LM, Zhou XE, Soon FF, Xu Y, Suino-Powell KM, Park SY, Weiner JJ, Fujii H, Chinnusamy V, Kovach A, Li J, Wang Y, Peterson FC, Jensen DR, Yong EL, Volkman BF, Cutler SR, Zhu JK, Xu HE (2009) Nature 462:602–608
Soon FF, Ng LM, Zhou XE, West GM, Kovach A, Tan MHE, Suino-Powell KM, He Y, Xu Y, Chalmers MJ, Brunzelle JS, Zhang H, Yang H, Jiang H, Li J, Yong EL, Cutler S, Zhu JK, Griffin PR, Melcher K, Xu HE (2012) Science 335:85–88
Jackson MD, Fjeld CC, Denu JM (2003) Biochemistry 42:8513–8521
Martinez MA, Das K, Saikolappan S, Materon LA, Dhandayuthapani S (2013) BMC Microbiol 13:44
Acknowledgments
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa Científica de Tecnológica do Estado de Santa Catarina (FAPESC), Financiadora de Estudos e Projetos (FINEP), and Instituto Nacional de Biologia Estrutural e Bioimagem (INBEB). We acknowledge the technical staff of CEBIME for assistance. We thank Maria Lucia Bianconi (UFRJ), Douglas Norberto, and Leonardo Rosado for helpful discussions about ITC data, and Catia Silene Klein for the M. synoviae strain 53 cells.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Menegatti, A.C.O., Vernal, J. & Terenzi, H. The unique serine/threonine phosphatase from the minimal bacterium Mycoplasma synoviae: biochemical characterization and metal dependence. J Biol Inorg Chem 20, 61–75 (2015). https://doi.org/10.1007/s00775-014-1209-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-014-1209-3