Abstract
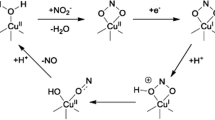
The high-yield expression and purification of Shewanella oneidensis cytochrome c nitrite reductase (ccNiR) and its characterization by a variety of methods, notably Laue crystallography, are reported. A key component of the expression system is an artificial ccNiR gene in which the N-terminal signal peptide from the highly expressed S. oneidensis protein “small tetraheme c” replaces the wild-type signal peptide. This gene, inserted into the plasmid pHSG298 and expressed in S. oneidensis TSP-1 strain, generated approximately 20 mg crude ccNiR per liter of culture, compared with 0.5–1 mg/L for untransformed cells. Purified ccNiR has nitrite and hydroxylamine reductase activities comparable to those previously reported for Escherichia coli ccNiR, and is stable for over 2 weeks in pH 7 solution at 4 °C. UV/vis spectropotentiometric titrations and protein film voltammetry identified five independent one-electron reduction processes. Global analysis of the spectropotentiometric data also allowed determination of the extinction coefficient spectra for the five reduced ccNiR species. The characteristics of the individual extinction coefficient spectra suggest that, within each reduced species, the electrons are distributed among the various hemes, rather than being localized on specific heme centers. The purified ccNiR yielded good-quality crystals, with which the 2.59-Å-resolution structure was solved at room temperature using the Laue diffraction method. The structure is similar to that of E. coli ccNiR, except in the region where the enzyme interacts with its physiological electron donor (CymA in the case of S. oneidensis ccNiR, NrfB in the case of the E. coli protein).










Similar content being viewed by others
Abbreviations
- ccNiR:
-
Cytochrome c nitrite reductase
- CCP4:
-
Collaborative Computational Project Number 4
- HAO:
-
Hydroxylamine oxidoreductase
- HEPES:
-
N-(2-Hydroxyethyl)piperazine-N′-ethanesulfonic acid
- MV:
-
Methyl viologen
- MVred :
-
Methyl viologen monocation radical
- OTTLE:
-
Optically transparent thin-layer electrode
- PFV:
-
Protein film voltammetry
- SHE:
-
Standard hydrogen electrode
- STC:
-
Small tetraheme c
- SVD:
-
Singular value decomposition
References
Schmidt I, Steenbakkers PJM, Op den Camp HJM, Schmidt K, Jetten MSM (2004) J Bacteriol 186:2781–2788. doi:10.1128/Jb.186.9.2781-2788.2004
Ehrlich HL (2002) Geomicrobiology. Marcel Dekker, New York
Einsle O, Messerschmidt A, Stach P, Bourenkov GP, Bartunik HD, Huber R, Kroneck PMH (1999) Nature 400:476–480
Einsle O, Stach P, Messerschmidt A, Simon J, Kroger A, Huber R, Kroneck PMH (2000) J Biol Chem 275:39608–39616
Pereira I, LeGall J, Xavier A, Teixeira M (2000) Biochim Biophys Acta 1481:119–130
Bamford VA, Angove HC, Seward HE, Thomson AJ, Cole JA, Butt JN, Hemmings AM, Richardson DJ (2002) Biochemistry 41:2921–2931. doi:10.1021/Bi015765d
Cunha CA, Macieira S, Dias JM, Almeida G, Goncalves LL, Costa C, Lampreia J, Huber R, Moura JJG, Moura I, Romao MJ (2003) J Biol Chem 278:17455–17465. doi:10.1074/jbc.M211777200
Rodrigues M, Oliveira T, Pereira I, Archer M (2006) EMBO J 25:5951–5960
Rodrigues ML, Oliveira T, Matias PM, Martins C, Valente FM, Pereira IA, Archer M (2006) Acta Crystallogr F 62:565–568
Igarashi N, Moriyama H, Fujiwara T, Fukumori Y, Tanaka N (1997) Nat Struct Biol 4:276–284
Einsle O, Messerschmidt A, Huber R, Kroneck PMH, Neese F (2002) J Am Chem Soc 124:11737–11745. doi:10.1021/Ja0206487
Kostera J, Youngblut MD, Slosarczyk JM, Pacheco AA (2008) J Biol Inorg Chem 13:1073–1083. doi:10.1007/s00775-008-0393-4
Kostera J, McGarry JM, Pacheco AA (2010) Biochemistry 49:8546–8553
Poock S, Leach E, Moir J, Cole J, Richardson D (2002) J Biol Chem 277:23664–23669
Lukat P, Rudolf M, Stach P, Messerschmidt A, Kroneck P, Simon J, Einsle O (2008) Biochemistry 47:2080–2086
Kurnikov IV, Ratner MA, Pacheco AA (2005) Biochemistry 44:1856–1863. doi:10.1021/Bi048060v
Bykov D, Neese F (2011) J Biol Inorg Chem 16:417–430
Ford PC, Lorkovic IM (2002) Chem Rev 102:993–1017. doi:10.1021/Cr0000271
Wolak M, van Eldik R (2002) Coord Chem Rev 230:263–282
Ravelli RBG, McSweeney SM (2000) Structure 8:315–328
Pearson AR, Pahl R, Kovaleva EG, Davidson VL, Wilmot CM (2007) J Synchrotron Radiat 14:92–98
Beitlich T, Kuhnel K, Schulze-Briese C, Shoeman RL, Schlichting I (2007) J Synchrotron Radiat 14:11–23
Moffat K, Szebenyi D, Bilderback D (1984) Science 223:1423–1425
Srajer V, Teng T, Ursby T, Pradervand C, Ren Z, Adachi S, Schildkamp W, Bourgeois D, Wulff M, Moffat K (1996) Science 274:1726–1729
Ren Z, Bourgeois D, Helliwell JR, Moffat K, Srajer V, Stoddard BL (1999) J Synchrotron Radiat 6:891–917
Schmidt M, Rajagopal S, Ren Z, Moffat K (2003) Biophys J 84:2112–2129
Schmidt M, Pahl R, Srajer V, Anderson S, Ren Z, Ihee H, Rajagopal S, Moffat K (2004) Proc Natl Acad Sci USA 101:4799–4804
Rajagopal S, Schmidt M, Anderson S, Moffat K (2004) Acta Crystallogr D 60:860–871
Schmidt M, Pahl R, Ihee H, Srajer V (2005) In: Nienhaus GU (ed) Protein–ligand interactions: methods and applications. Humana Press, Totowa
Rajagopal S, Anderson S, Srajer V, Schmidt M, Pahl R, Moffat K (2005) Structure 13:55–63
Ihee H, Rajagopal S, Srajer V, Pahl R, Schmidt M, Schotte F, Anfinrud PA, Wulff M, Moffat K (2005) Proc Natl Acad Sci USA 102:7145–7150
Key J, Srajer V, Pahl R, Moffat K (2007) Biochemistry 46:4706–4715
Schmidt M (2008) In: Zinth W, Braun M, Gilch P (eds) Ultrashort laser pulses in medicine and biology. Springer, Berlin
Raicu V, Schmidt M, Stoneman M (2009) In: Jakovlev V (ed) Biochemical applications of nonlinear optical spectroscopy. CRC Press, FL pp 1–32
Schmidt M, Šrajer V, Purwar N, Tripathi S (2012) J Synchrotron Radiat 19:264–273 doi:10.1107/S090904951105549X
Tripathi S, Srajer V, Purwar N, Henning RW, Schmidt M (2012) Biophys J 102:325–332
Tsapin AI, Nealson KH, Meyers T, Cusanovich MA, van Beuumen J, Crosby LD, Feinberg BA, Zhang C (1996) J Bacteriol 178:6386–6388
Takayama Y, Akutsu H (2007) Protein Expr Purif 56:80–84
Ozawa K, Yasukawa F, Fujiwara Y, Akutsu H (2001) Biosci Biotechnol Biochem 65:185–189
Dawson RMC, Elliot DC, Jones KM (1969) Data for biochemical research, Oxford University Press, London
Atkinson SJ, Mowat CG, Reid GA, Chapman SK (2007) FEBS Lett 581:3805–3808
Heineman WR, Norris BJ, Goelz JF (1975) Anal Chem 47:79–84
Pilkington MBG, Coles BA, Compton RG (1989) Anal Chem 61:1787–1789
Bodemer GJ (2008) PhD dissertation. University of Wisconsin-Milwaukee, Milwaukee
Watanabe T, Honda K (1982) J Phys Chem 86:2617–2619
Fourmond V, Hoke K, Heering HA, Baffert C, Leroux F, Bertrand P, Leger C (2009) Bioelectrochemistry 76:141–147
Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AGW (2011) Acta Crystallogr D 67:271–281. doi:10.1107/S0907444910048675
Collaborative Computational Project Number 4 (1994) Acta Crystallogr D 50:760–763
Graber T, Anderson S, Brewer H, Chen YS, Cho HS, Dashdorj N, Henning RW, Kosheleva I, Macha G, Meron M, Pahl R, Ren Z, Ruan S, Schotte F, Rajer VS, Viccaro PJ, Westferro F, Anfinrud P, Moffat K (2011) J Synchrotron Radiat 18:658–670
Press WH, Teukolsky SA, Vetterling WT, Flannery BP (2007) Numerical recipes, 3rd edn. Cambridge University Press, New York, pp 65–75 and 773–839
Henry ER, Hofrichter J (1992) In: Brand L, Johnson ML (eds) Methods in enzymology. Academic Press, San Diego, pp 129–192
Gates AJ, Kemp GL, To CY, Mann J, Marritt SJ, Mayes AG, Richardson DJ, Butt JN (2011) Phys Chem Chem Phys 13:7720–7731
McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ (2002) J Appl Crystallogr 40:658–674
Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Acta Crystallogr D 66:486–501
Thony-Meyer L, Fischer F, Kunzler P, Ritz D, Hennecke H (1995) J Bacteriol 177:4321–4326
Londer Y, Giuliani SE, Peppler T, Collart FR (2008) Protein Expr Purif 62:128–137
Londer YY, Pokkuluri PR, Tiede DM, Schiffer M (2002) Biochim Biophys Acta 1554:202–211
Londer YY, Pokkuluri PR, Erickson J, Orshonsky V, Schiffer M (2005) Protein Expr Purif 39:254–260
Londer YY, Pokkuluri PR, Orshonsky V, Orshonsky L, Schiffer M (2006) Protein Expr Purif 47:241–248
Eaves DJ, Grove J, Staudenmann W, James P, Poole RK, White SA, Griffiths L, Cole JA (1998) Mol Microbiol 28:205–216
Ozawa K, Tsapin AI, Nealson KH, Cusanovich MA, Akutsu H (2000) Appl Environ Microbiol 66:4168–4171
Shi L, Lin JT, Markillie LM, Squier TC, Hooker BS (2005) Biotechniques 38:297–299
Shi L, Chen B, Wang Z, Elias DA, Mayer MU, Gorby YA, Ni S, Lower BH, Kennedy DW, Wunschel DS, Mottaz HM, Marshall MJ, Hill EA, Beliaev AS, Zachara JM, Fredrickson JK, Squier TC (2006) J Bacteriol 188:4705–4714
Marritt SJ, Kemp GL, Xiaoe L, Durrant JR, Cheesman MR, Butt JN (2008) J Am Chem Soc 130:8588–8589
Kiefersauer R, Than ME, Dobbek H, Gremer L, Melero M, Strobl S, Dias JM, Soulimane T, Huber R (2000) J Appl Crystallogr 33:1223–1230
Southworth-Davies RJ, Medina MA, Carmichael I, Garman EF (2007) Structure 15:1531–1541
Rajendran C, Dworkowski FS, Wang M, Schulze-Briese C (2011) J Synchrotron Radiat 18:318–328
Walker FA (1999) Coord Chem Rev 186:471–534
Clarke TA, Kemp GL, Van Wonderen JH, Doyle RAS, Cole JA, Tovell N, Cheesman MR, Butt JN, Richardson DJ, Hemmings AM (2008) Biochemistry 47:3789–3799
Stach P, Einsle O, Schumacher W, Kurun E, Kroneck PMH (2000) J Inorg Biochem 79:381–385
Holm L, Park J (2000) Bioinformatics 16:566–567
Gao H, Yang ZK, Barua S, Reed SB, Romine MF, Nealson KH, Fredrickson JK, Tiedje JM, Zhou J (2009) ISME J 3:966–976
Acknowledgments
M.S. and A.A.P. are supported by grants NSF-0843459, NSF-1121770, and UWM RGI 101X157. M.S. is supported by NSF CAREER grant 0952643. E.T.J. is supported by an NIH-NIGMS National Research Service Award (NRSA) fellowship (1F31GM099416-01). B.S. and T.G. gratefully acknowledge support from University of Wisconsin-Milwaukee’s Office of Undergraduate Research. Use of the BioCARS Sector 14 was supported by grants from the National Center for Research Resources (5P41RR007707) and the National Institute of General Medical Sciences (8P41GM103543) from the National Institutes of Health. The time-resolved setup at Sector 14 was funded in part through a collaboration with Philip Anfinrud (NIH/NIDDK). Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under contract no. DE-AC02-06CH11357. The authors gratefully acknowledge Nick Silvaggi, Chuck Wimpee, Graham Moran, and Daad Safarini of the University of Wisconsin-Milwauke for helpful discussions, Victor Trussell of Rufus King High School for help in stability studies of ccNiR, Nick Silvaggi for the use of his high-performance chromatography apparatus, and the Jianhua Fu research group (Medical College of Wisconsin) for the use of their automated crystallization machine in the preliminary crystallization screens.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Youngblut, M., Judd, E.T., Srajer, V. et al. Laue crystal structure of Shewanella oneidensis cytochrome c nitrite reductase from a high-yield expression system. J Biol Inorg Chem 17, 647–662 (2012). https://doi.org/10.1007/s00775-012-0885-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-012-0885-0