Abstract
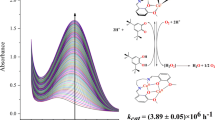
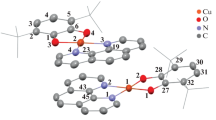
The monohydroxo-bridged dicopper(II) complex (1), its reduced dicopper(I) analogue (2) and the trans-μ-1,2-peroxo-dicopper(II) adduct (3) with the macrocyclic N-donor ligand [22]py4pz (9,22-bis(pyridin-2′-ylmethyl)-1,4,9,14,17,22,27,28,29,30- decaazapentacyclo -[22.2.114,7.111,14.117,20]triacontane-5,7(28),11(29),12,18,20(30), 24(27),25-octaene), have been prepared and characterized, including a 3D structure of 1 and 2. These compounds represent models of the three states of the catechol oxidase active site: met, deoxy (reduced) and oxy. The dicopper(II) complex 1 catalyzes the oxidation of catechol model substrates in aerobic conditions, while in the absence of dioxygen a stoichiometric oxidation takes place, leading to the formation of quinone and the respective dicopper(I) complex. The catalytic reaction follows a Michaelis–Menten behavior. The dicopper(I) complex binds molecular dioxygen at low temperature, forming a trans-μ-1,2-peroxo-dicopper adduct, which was characterized by UV–Vis and resonance Raman spectroscopy and electrochemically. This peroxo complex stoichiometrically oxidizes a second molecule of catechol in the absence of dioxygen. A catalytic mechanism of catechol oxidation by 1 has been proposed, and its relevance to the mechanisms earlier proposed for the natural enzyme and other copper complexes is discussed.












Similar content being viewed by others
References
Solomon EI, Sundaram UM, Machonkin TE (1996) Chem Rev 96:2563–2605
Magnus KA, Ton-That H, Carpenter JE (1993) In: Karlin KD, Tyeklar Z (eds) Bioinorganic Chemistry of Copper. Chapman&Hall, New York, pp 143–150
Volbeda A, Hol WG (1989) J Mol Biol 209:249–279
Klabunde T, Eicken C, Sacchettini JC, Krebs B (1998) Nat Struct Biol 5:1084–1090
Selmeczi K, Reglier M, Speier G, Peintler G (2004) React Kinet Catal Lett 81:143–151
Selmeczi K, Reglier M, Giorgi M, Speier G (2003) Coord Chem Rev 245: 191–201
Gao J, Reibenspies JH, Martell AE (2003) Inorg Chim Acta 346:67–75
Ackermann J, Meyer F, Kaifer E, Pritzkow H (2002) Chem Eur J 8: 247–258
Börzel H, Comba P, Pritzkow H (2001) Chem Commun 97–98
Granata A, Monzani E, Casella L (2004) J Biol Inorg Chem 9:903–913
Louloudi M, Mitopoulou K, Evaggelou E, Deligiannakis Y, Hadjiliadis N (2003) J Mol Cat A 198:231–240
Merkel M, Möller N, Piacenza M, Grimme S, Rompel A, Krebs B (2005) Chem Eur J 11:1201–1209
Mukherjee J, Mukherjee R (2002) Inorg Chim Acta 337:429–438
Monzani E, Battaini G, Perotti A, Casella L, Gullotti M, Santagostini L, Nardin G, Randaccio L, Geremia S, Zanello P, Opromolla G (1999) Inorg Chem 38:5359–5369
Monzani E, Quinti L, Perotti A, Casella L, Gulotti M, Randaccio L, Geremia S, Nardin G, Faleschini P, Tabbi G (1998) Inorg Chem 37:553–562
Than R, Feldmann AA, Krebs B (1999) Coord Chem Rev 182:211–241
Torelli S, Belle C, Gautier-Luneau I, Pierre JL, Saint-Aman E, Latour JM, Le Pape L, Luneau D (2000) Inorg Chem 39:3526–3536
Torelli S, Belle C, Hamman S, Pierre JL, Saint-Aman E (2002) Inorg Chem 41:3983–3989
Koval IA, van der Schilden K, Schuitema AM, Gamez P, Belle C, Pierre J-L, Luken M, Krebs B, Roubeau O, Reedijk J (2005) Inorg Chem 44: 4372–4382
Gerdemann C, Eicken C, Krebs B (2002) Acc Chem Res 35:183–191
Schuitema AM, Aubel PG, Koval IA, Engelen M, Driessen WL, Reedijk J, Lutz M, Spek AL (2003) Inorg Chim Acta 355:374–385
Schwarzenbach G, Schwarzenbach K (1963) Helv Chim Acta 46: 1390–1400
Casella L, Monzani E, Gulotti M, Cavagnino D, Cerina G, Santagostini L, Ugo R (1996) Inorg Chem 35:7516–7525
Battaini G, Monzani E, Casella L, Santagostini L, Pagliarin R (2000) J Biol Inorg Chem 5:262–268
Battaini G, De Carolis M, Monzani E, Tuczek F, Casella L (2003) Chem Commun:726–727
TEXSAN, Single-crystal structure analysis software, version 1.7 (1995) Molecular Structure Corporation:The Woodlands, TX
Addison AW, Rao TN, Reedijk J, van Rijn J, Verschoor GC (1984) J Chem Soc, Dalton Trans 1349–1356
Liu W, Thorp HH (1993) Inorg Chem 32:4102–4105
Baldwin MJ, Ross PK, Pate JE, Tyeklár Z, Karlin KD, Solomon EI (1991) J Am Chem Soc 113:8671–8679
Solomon EI, Tuczek F, Root DE, Brown CA (1994) Chem Rev 94:827–856
Mirica LM, Ottenwaelder X, Stack TDP (2004) Chem Rev 104:1013–1045
Bol JE, Driessen WL, Ho RYN, Maase B, Que L, Reedijk J (1997) Ang Chem, Int Ed 36:998–1000
Gamez P, Koval IA, Reedijk J (2004) Dalton Transactions:4079–4088
Paul PP, Tyeklár Z, Jacobson RR, Karlin KD (1991) J Am Chem Soc 113:5322–5332
Reim J, Krebs B (1997) J Chem Soc, Dalton Trans 3793–3804
Shearer J, Xin Zhang C, Zacharov LN, Rheingold AL, Karlin KD (2005) J Am Chem Soc 127:5469–5483
Ghiladi M, Gomez JT, Hazell A, Lumtscher J, McKenzie CJ (2003) Dalton Trans 1320–1325
Harris WR, McLendon GL, Martell AE, Bess RC, Mason M (1980) Inorg Chem 19:21–26
Eicken C, Krebs B, Sacchettini JC (1999) Curr Opin Struct Biol 9: 677–683
Gupta M, Mathur P, Butcher RJ (2001) Inorg Chem 40:878–885
Rockcliffe DA, Martell AE (1995) J Mol Catal A 99:101–114
Chyn J-P, Urbach FL (1991) Inorg Chim Acta 189:157–163
Kitajima N, Koda T, Iwata Y, Moro-oka Y (1990) J Am Chem Soc 112: 8833–8839
Santagostini L, Gulotti M, Monzani E, Casella L, Dillinger R, Tuczek F (2000) Chem Eur J 6:519–522
Mahadevan V, Dubois L, Hedman B, Hodgson KO, Stack TD (1999) J Am Chem Soc 121:5583–5584
Berreau LM, Mahapatra S, Halfen JA, Houser RP, Young VG, Tolman WB (1999) Angew Chem Int Ed Engl 38:207–210
Rockcliffe DA, Martell AE (1992) J Chem Soc, Chem Commun 1758–1760
Rockcliffe DA, Martell AE, Reibenspies JH (1996) J Chem Soc, Dalton Trans 167–175
Rockcliffe DA, Martell AE (1996) J Mol Cat A 106:211–221
Rockcliffe DA, Martell AE (1995) J Mol Catal A 99:87–99
Rockcliffe DA, Martell AE (1993) Inorg Chem 32:3143–3152
Börzel H, Comba P, Hagen KS, Kerscher M, Pritzkow H, Schatz E, Schindler S, Walter O (2002) Inorg Chem 41:5440–5452
Root DE, Mahroof-Tahir M, Karlin KD, Solomon EI (1998) Inorg Chem 37:4838–4848
Itoh K, Hayashi H, Furutachi H, Mathumoto T, Nagamoto S, Tosha T, Terada S, Fujinami S, Suzuki F, Kitagawa T (2005) J Am Chem Soc, and references therein 127:5212–5223
Karlin KD, Ghosh P, Cruse RW, Meyer GJ, Farooq A, Gultneh Y, Jacobson RR, Blackburn NJ, Strange RW, Zubieta J (1988) J Am Chem Soc 110:6769–6780
Itoh S, Kumei H, Taki M, Nagamoto S, Kitagawa T, Fukuzumi S (2001) J Am Chem Soc 123:6708–6709
Martell AE, Motekaitis RJ, Menif R, Rockcliffe DA, Llobet A (1997) J Mol Cat A 117:205–213
Rompel A, Fischer H, Meiwes D, Buldt Karentsopoulos K, Dillinger R, Tuczek F, Witzel H, Krebs B (1999) J Biol Inorg Chem 4:56–63
Jung B, Karlin KD, Zuberbühler AD (1996) J Am Chem Soc 118:3763–3764
Acknowledgements
Support of the NRSC Catalysis (a Research School Combination of HRSMC and NIOK) is kindly acknowledged. Also support and sponsorship concerted by COST Action D21/003/2001 is gratefully acknowledged. Collaborative travel grant from the French Ministry of Research and Foreign Affairs (EGIDE) and NWO (Van Gogh Programme), allowing visits and exchanges between Leiden and Grenoble, is gratefully acknowledged. We are very much indebted to Prof. L. Casella and Dr. E. Monzani (University of Pavia, Italy) for their kind assistance with the monophenolase activity studies and for hosting one of the co-authors (AMS). Crystallographic data (without structure factors) for the structure of 2 reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-269469. Copies of the data can be obtained free of charge from the CCDC (12 Union Road, Cambridge CB2 1EZ, UK; tel: (+44) 1223-336-408; fax: (+44) 1223-336-003; e-mail: deposit@ccdc.cam.ac.uk; website see link below).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Koval, I.A., Belle, C., Selmeczi, K. et al. Catecholase activity of a μ-hydroxodicopper(II) macrocyclic complex: structures, intermediates and reaction mechanism. J Biol Inorg Chem 10, 739–750 (2005). https://doi.org/10.1007/s00775-005-0016-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-005-0016-2