Abstract
The existence of a link between some cases of familial amyotrophic lateral sclerosis (FALS) and copper-zinc superoxide dismutase (CuZnSOD) has been understood for almost a decade. However, beyond the fact that mutations in CuZnSOD cause FALS by a toxic gain of function, the mechanism whereby specific mutations in the protein structure result in development of the disease has remained almost a complete mystery to date. We have undertaken a critical survey of in vitro characteristics of over 30 of the 90 different CuZnSOD mutant proteins that are known to cause FALS in order to determine the differences that exist between mutant and wild-type properties. As-isolated metal content analysis, SOD activity assays, and thermal stability determinations of a significant fraction of the mutants show that the FALS mutant SOD proteins can be classified distinctly into one of two groups. Members of the first group, termed wild-type-like, have physical properties and enzymatic activities that are strikingly similar to those of wild-type CuZnSOD. The second group, however, show aberrant metal content in the as-isolated forms, compromised SOD activities, and unusual DSC thermoscans. All mutations in the members of this second group occur in or near the metal binding sites of the protein and thus they are termed metal binding region mutants. We have also compared the relative rates of self-inactivation caused by reaction of the wild-type protein and several FALS-linked CuZnSOD mutants with hydrogen peroxide, as a measure of relative peroxidative activities. Results and implications of the role of CuZnSOD in FALS are discussed.










Similar content being viewed by others
Abbreviations
- ALS:
-
amyotrophic lateral sclerosis
- DSC:
-
differential scanning calorimetry
- FALS:
-
familial ALS
- ICP-MS:
-
inductively coupled plasma mass spectrometry
- POBN:
-
α-(pyridyl 4-N-oxide) N-tert-butyl nitrone
- SOD1 or CuZnSOD:
-
copper-zinc superoxide dismutase protein, product of the sod1 gene
References
Smith RG, Appel SH (1995) Annu Rev Med 46:133–145
Cleveland DW, Rothstein JD (2001) Nat Rev Neurosci 2:806–819
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung WY, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH Jr (1993) Nature 362:59–62
Gaudette M, Hirano M, Siddique T (2000) Amyotroph Lateral Scler Other Motor Neuron Disord 1:83–89
Andersen PM, Nilsson P, Ala-Hurula V, Keranen ML, Tarvainen I, Haltia T, Nilsson L, Binzer M, Forsgren L, Marklund SL (1995) Nat Genet 10:61–66
Fridovich I (1997) J Biol Chem 272:18515–18517
Ellerby LMC, Cabelli DE, Graden JA, Valentine JS (1996) J Am Chem Soc 118:6556–6561
Klug D, Rabani J, Fridovich I (1972) J Biol Chem 247:4839–4842
Rotilio G, Bray RC, Fielden EM (1972) Biochim Biophys Acta 268:605–609
Julien JP (2001) Cell 104:581–591
Watanabe M, Dykes-Hoberg M, Culotta VC, Price DL, Wong PC, Rothstein JD (2001) Neurobiol Dis 8:933–941
Matsumoto S, Kusaka H, Ito H, Shibata N, Asayama T, Imai T (1996) Clin Neuropathol 15:41–46
Shibata N, Asayama K, Hirano A, Kobayashi M (1996) Dev Neurosci 18:492–498
Shibata N, Hirano A, Kobayashi M, Sasaki S, Kato T, Matsumoto S, Shiozawa Z, Komori T, Ikemoto A, Umahara T, Asayama K (1994) Neurosci Lett 179:149–152
Johnston JA, Dalton MJ, Gurney ME, Kopito RR (2000) Proc Natl Acad Sci USA 97:12571–12576
Valentine JS (2003) Free Radical Biol Med (in press)
Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH Jr, Scott RW, Snider WD (1996) Nat Genet 13:43–47
Gurney ME (1997) J Neurol (Suppl 2) 244:S15–S20
Dal Canto MC, Gurney ME (1997) Acta Neuropathol 93:537–550
Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen WJ, Zhai P, Sufit RL, Siddique T (1994) Science 264:1772–1775
Steinkühler C, Sapora O, Carri MT, Nagel W, Marcocci L, Ciriolo MR, Weser U, Rotilio G (1991) J Biol Chem 266:24580–24587
Galiazzo F, Ciriolo MR, Carri MT, Civitareale P, Marcocci L, Marmocchi F, Rotilio G (1991) Eur J Biochem 196:545–549
Estevez AG, Crow JP, Sampson JB, Reiter C, Zhuang Y, Richardson GJ, Tarpey MM, Barbeito L, Beckman JS (1999) Science 286:2498–2500
Hayward LJ, Rodriguez JA, Kim JW, Tiwari A, Goto JJ, Cabelli DE, Valentine JS, Brown RH Jr (2002) J Biol Chem 277:15923–15931
Rodriguez JA, Valentine JS, Eggers DK, Roe JA, Tiwari A, Brown RH Jr, Hayward LJ (2002) J Biol Chem 277:15932–15937
Rabizadeh S, Gralla EB, Borchelt DR, Gwinn R, Valentine JS, Sisodia S, Wong P, Lee M, Hahn H, Bredesen DE (1995) Proc Natl Acad Sci USA 92:3024–3028
Wiedau-Pazos M, Goto JJ, Rabizadeh S, Gralla EB, Roe JA, Lee MK, Valentine JS, Bredesen DE (1996) Science 271:515–518
Goto JJ, Gralla EB, Valentine JS, Cabelli DE (1998) J Biol Chem 273:30104–30109
Malek K (2002) PhD thesis, University of California, Los Angeles
Goto JJ, Zhu H, Sanchez RJ, Nersissian A, Gralla EB, Valentine JS, Cabelli DE (2000) J Biol Chem 275:1007–1014
Roe JA, Wiedau-Pazos M, Moy VN, Goto JJ, Gralla EB, Valentine JS (2002) Free Radical Biol Med 32:169–174
Liu R, Althaus JS, Ellerbrock BR, Becker DA, Gurney ME (1998) Ann Neurol 44:763–770
Bogdanov MB, Ramos LE, Xu Z, Beal MF (1998) J Neurochem 71:1321–1324
Andrus PK, Fleck TJ, Gurney ME, Hall ED (1998) J Neurochem 71:2041–2048
Kurahashi T, Miyazaki A, Suwan S, Isobe M (2001) J Am Chem Soc 123:9268–9278
Acknowledgements
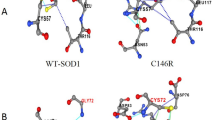
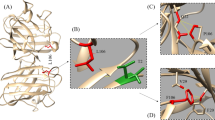
We thank Jorge Rodriguez and Dr. Edith Gralla for helpful comments on this manuscript. We also thank Dr. P. John Hart at the University of Texas Health Science Center at San Antonio and Dr. Lawrence Hayward at the University of Massachusetts Medical School for many scientific discussions about ALS and SOD. Figures 2 and 3 were kindly provided by Dr. P. John Hart. This work was supported by grants from the ALS Association and National Institutes of Health Grant GM28222.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Potter, S.Z., Valentine, J.S. The perplexing role of copper-zinc superoxide dismutase in amyotrophic lateral sclerosis (Lou Gehrig's disease). J Biol Inorg Chem 8, 373–380 (2003). https://doi.org/10.1007/s00775-003-0447-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-003-0447-6