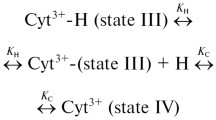Abstract.
Thermal denaturation of the mesophilic rubredoxin from Clostridium pasteurianum occurs through a number of temperature-dependent steps, the last and irreversible one being release of iron from the [Fe2+(SCys)4] site. We show here that thermally induced [Fe2+(SCys)4] site destruction is largely determined by the local environment, and not directly connected to thermostability of the native polypeptide fold of rubredoxin. Hydrophobic residues on the protein surface, V8 and L41, that shield the [Fe(SCys)4] site from solvent and form N-H...S hydrogen bonds to the metal-coordinating sulfurs, were mutated to residues with both uncharged and charged side chains. On these mutated rubredoxins the temperature dependence was measured for: (1) global unfolding of the protein by NMR, (2) loss of Fe2+ at various ionic strengths and pH values, (3) the rates of non-denaturing displacement of Fe2+ by Cd2+ or Zn2+. For reversible temperature-dependent changes in the global protein folding that occur prior to loss of iron, no thermostability differences were found among the wild-type, V8A, V8D, L41R, and L41D rubredoxins. However, for irreversible loss of iron from the [Fe2+(SCys)4] site, relative to the wild-type protein, L41R was more thermostable, V8A was somewhat less thermostable, and the acidic mutants L41D, V8D and [V8D, L41D] showed dramatically lowered thermostability. Lower pH facilitated – both kinetically and thermodynamically – thermally induced iron release, likely through protonation of ligand cysteines' thiols. For all of the rubredoxins a direct correlation was found between the midpoint temperature for thermally induced Fe2+ loss and the rate of non-denaturing Fe2+ displacement by Cd2+ or Zn2+ at room temperature. A mechanism is proposed involving transient movement of residue-8 and -41 side chains, allowing, and, in the case of negatively charged side chains, also facilitating, attack of a ligand cysteine by the incoming positively charged species (H+, Cd2+, or Zn2+). Thus, localized charge density and solvent accessibility modulate the stability of Fe2+ ligation in rubredoxin. However, the reduced [Fe(SCys)4] site does not control the thermostability of the native polypeptide fold of rubredoxin.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Bonomi, F., Burden, A.E., Eidsness, M.K. et al. Thermal stability of the [Fe(SCys)4] site in Clostridium pasteurianum rubredoxin: contributions of the local environment and Cys ligand protonation. J Biol Inorg Chem 7, 427–436 (2002). https://doi.org/10.1007/s00775-001-0314-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-001-0314-2