Abstract
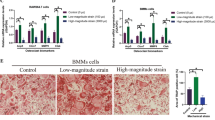
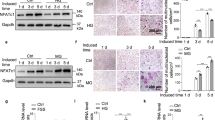
Little is known about the effects of tensile forces on osteoclastogenesis by human monocytes in the absence of mechanosensitive cells, including osteoblasts and fibroblasts. In this study we consider the effects of tensile force on osteoclastogenesis in human monocytes. The cells were treated with receptor activator of nuclear factor κB ligand (RANKL) to promote osteoclastogenesis. Then,expression and secretion of cathepsin K were examined. RANKL and the formation of osteoclasts during the osteoclast differentiation process under continual tensile stress were evaluated by Western blot. It was also found that −100 kPa or lower induces RANKL-enhanced tartrate-resistant acid phosphatase activity in a dose-dependent manner. Furthermore, an increased tensile force raises the expression and secretion of cathepsin K elevated by RANKL, and is concurrent with the increase of TNF-receptor-associated factor 6 induction and nuclear factor κB activation. Overall, the current report demonstrates that tensile force reinforces RANKL-induced osteoclastogenesis by retarding osteoclast differentiation. The tensile force is able to modify every cell through dose-dependent in vitro RANKL-mediated osteoclastogenesis, affecting the fusion of preosteoclasts and function of osteoclasts. However, tensile force increased TNF-receptor-associated factor 6 expression. These results are in vitro findings and were obtained under a condition of tensile force. The current results help us to better understand the cellular roles of human macrophage populations in osteoclastogenesis as well as in alveolar bone remodeling when there is tensile stress.







Similar content being viewed by others
References
Eriksen EF (2010) Cellular mechanisms of bone remodeling. Rev Endocr Metab Disord 11:219–227
Terai K, Takano-Yamamoto T, Ohba Y, Hiura K, Sugimoto M et al (1999) Role of osteopontin in bone remodeling caused by mechanical stress. J Bone Miner Res 14:839–849
Wise GE, Yao S (2003) Expression of tumour necrosis factor-alpha in the rat dental follicle. Arch Oral Biol 48:47–54
Nayak BN, Wiltshire WA, Ganss B, Tenenbaum H, McCulloch CAG et al (2008) Healing of periodontal tissues following transplantation of cells in a rat orthodontic tooth movement model. Angle Orthod 78:826–831
Huang TH, Lu YC, Kao CT (2012) Low-level diode laser therapy reduces lipopolysaccharide (LPS)-induced bone cell inflammation. Lasers Med Sci 27:621–627
Chen YJ, Shie MY, Hung CJ, Wu BC, Liu SL et al (2013) Activation of focal adhesion kinase induces extracellular signal- regulated kinase-mediated osteogenesis in tensile force-subjected periodontal ligament fibroblasts but not in osteoblasts. J Bone Miner Metab 32:671–682
Kuo CL, Kao CT, Fang HY, Huang TH, Chen YW et al (2015) Antiosteoclastogenesis activity of a CO2 laser antagonizing receptor activator for nuclear factor kappaB ligand-induced osteoclast differentiation of murine macrophages. Laser Phys Lett 12:035681
Krishnan V, Davidovitch Z (2006) Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop 129:469.e1–469.e32
Wescott DC, Pinkerton MN, Gaffey BJ, Beggs KT, Milne TJ et al (2007) Osteogenic gene expression by human periodontal ligament cells under cyclic tension. J Dent Res 86:1212–1216
Hsieh WH, Chen YJ, Hung CJ, Huang TH, Kao CT et al (2014) Osteogenesis differentiation of human periodontal ligament cells by CO2 laser-treatment stimulating macrophages via BMP2 signalling pathway. Laser Phys 24:115607
Singh PP, van der Kraan AGJ, Xu J, Gillespie MT, Quinn JMW (2012) Membrane-bound receptor activator of NFκB ligand (RANKL) activity displayed by osteoblasts is differentially regulated by osteolytic factors. Biochem Biophys Res Commun 422:48–53
Chen YJ, Shie MY, Hung CJ, Liu SL, Huang TH et al (2015) Osteoblasts subjected to tensile force inducing osteoclastic differentiation of murine macrophage in a co-culture system. J Dent Sci 10:81–87
Lekic P, McCulloch CAG (1996) Periodontal ligament cell populations: the central role of fibroblasts in creating a unique tissue. Anat Rec 245:327–341
Batra NN, Li YJ, Yellowley CE, You L, Malone AM et al (2005) Effects of short-term recovery periods on fluid-induced signaling in osteoblastic cells. J Biomech 38:1909–1917
Liedert A, Kaspar D, Blakytny R, Claes L, Ignatius A (2006) Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun 349:1–5
Mayahara K, Yamaguchi A, Takenouchi H, Kariya T, Taguchi H et al (2012) Osteoblasts stimulate osteoclastogenesis via RANKL expression more strongly than periodontal ligament cells do in response to PGE2. Arch Oral Biol 57:1377–1384
Hamamura K, Tanjung N, Yokota H (2013) Suppression of osteoclastogenesis through phosphorylation of eukaryotic translation initiation factor 2 alpha. J Bone Miner Metab 31:618–628. doi:10.1007/s00774-013-0450-0
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055
Tang Y, Sun F, Li X, Zhou YZ, Yin S et al (2011) Porphyromonas endodontalis lipopolysaccharides induce RANKL by mouse osteoblast in a way different from that of Escherichia coli lipopolysaccharide. J Endod 37:1653–1659
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E et al (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Matsuo K, Irie N (2008) Osteoclast–osteoblast communication. Arch Biochem Biophys 473:201–209
Asagiri M, Takayanagi H (2007) The molecular understanding of osteoclast differentiation. Bone 40:251–264
Guihard P, Danger Y, Brounais B, David E, Brion R et al (2012) Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 30:762–772
Alexander KA, Chang MK, Maylin ER, Kohler T, Müller R et al (2011) Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res 26:1517–1532
Kook SH, Lee JC (2012) Tensile force inhibits the proliferation of human periodontal ligament fibroblasts through Ras-p38 MAPK up-regulation. J Cell Physiol 227:1098–1106
Castillo AB, Blundo JT, Chen JC, Lee KL, Yereddi NR et al (2012) Focal adhesion kinase plays a role in osteoblast mechanotransduction In vitro but does not affect load-induced bone formation in vivo. PLoS One 7:e43291
Zhang S, Cheng J, Qin YX (2012) Mechanobiological modulation of cytoskeleton and calcium influx in osteoblastic cells by short-term focused acoustic radiation force. PLoS One 7:e38343
Gould R, Chin K, Santisakultarm T, Dropkin A, Richards J et al (2012) Cyclic strain anisotropy regulates valvular interstitial cell phenotype and tissue remodeling in three-dimensional culture. Acta Biomater 8:1710–1719
Henriksen K, Neutzsky-Wulff AV, Bonewald LF, Karsdal MA (2009) Local communication on and within bone controls bone remodeling. Bone 44:1026–1033
Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 12:17–25
Zheng L, Huang Y, Song W, Gong X, Liu M et al (2012) Fluid shear stress regulates metalloproteinase-1 and 2 in human periodontal ligament cells: involvement of extracellular signal-regulated kinase (ERK) and P38 signaling pathways. J Biomech 45:2368–2375
Tan SD, Kuijpers-Jagtman AM, Semeins CM, Bronckers ALJJ, Maltha JC et al (2006) Fluid shear stress inhibits TNFα-induced osteocyte apoptosis. J Dent Res 85:905–909
Kim SJ, Park KH, Park YG, Lee SW, Kang YG (2013) Compressive stress induced the up-regulation of M-CSF, RANKL, TNF-α expression and the down-regulation of OPG expression in PDL cells via the integrin-FAK pathway. Arch Oral Biol 58:707–716
Yan YX, Gong YW, Guo Y, Lv Q, Guo C et al (2012) Mechanical strain regulates osteoblast proliferation through integrin-mediated ERK activation. PLoS One 7:e35709
Steward A, Thorpe S, Vinardell T, Buckley C, Wagner D et al (2012) Cell–matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomater 8:2153–2159
Kurata K, Uemura T, Nemoto A, Tateishi T, Murakami T et al (2001) Mechanical strain effect on bone-resorbing activity and messenger RNA expressions of marker enzymes in isolated osteoclast culture. J Bone Miner Res 16:722–730
Rubin J, Rubin C, Jacobs CR (2006) Molecular pathways mediating mechanical signaling in bone. Gene 367:1–16
Cho ES, Lee KS, Son YO, Jang YS, Lee SY et al (2010) Compressive mechanical force augments osteoclastogenesis by bone marrow macrophages through activation of c-Fms-mediated signaling. J Cell Biochem 111:1260–1269
Inaoka T, Bilbe G, Ishibashi O, Tezuka K, Kumegawa M et al (1995) Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun 206:89–96
Ravn P, Clemmesen B, Christiansen C (1999) Biochemical markers can predict the response in bone mass during alendronate treatment in early postmenopausal women. Bone 24:237–244
Gelb BD, Shi GP, Chapman HA, Desnick RJ (1996) Pycnodysostosis, a lysosomal disease caused by cathepsin K deficiency. Science 273:1236–1238
Hung CJ, Kao CT, Chen YJ, Shie MY, Huang TH (2013) Antiosteoclastogenic activity of silicate-based materials antagonizing receptor activator for nuclear factor kappaB ligand–induced osteoclast differentiation of murine marcophages. J Endod 39:1557–1561
Kook SH, Son YO, Hwang J-M, Kim E-M, Lee C-B et al (2009) Mechanical force inhibits osteoclastogenic potential of human periodontal ligament fibroblasts through OPG production and ERK-mediated signaling. J Cell Biochem 106:1010–1019
Takayanagi H (2005) Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med 83:170–179
Asagiri M, Sato K, Usami T, Ochi S, Nishina H et al (2005) Autoamplification of NFATc1 expression determines its essential role in bone homeostasis. J Exp Med 202:1261–1269
Feng H, Cheng T, Steer JH, Joyce DA, Pavlos NJ et al (2009) Myocyte enhancer factor 2 and microphthalmia-associated transcription factor cooperate with NFATc1 to transactivate the V-ATPase d2 promoter during RANKL-induced osteoclastogenesis. J Biol Chem 284:14667–14676
Acknowledgments
The authors acknowledge receipt of a grant from the National Science Council (MOST 102-2314-B-040-007-MY3) of Taiwan.
Conflict of interest
The authors declare that they have no conflict of interest and that they have no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Kao, CT., Huang, TH., Fang, HY. et al. Tensile force on human macrophage cells promotes osteoclastogenesis through receptor activator of nuclear factor κB ligand induction. J Bone Miner Metab 34, 406–416 (2016). https://doi.org/10.1007/s00774-015-0690-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-015-0690-2