Abstract
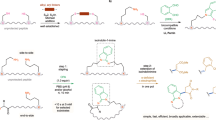
Selective removal of protecting groups under different cleavage mechanisms could be an asset in peptide synthesis, since it provides the feasibility to incorporate different functional groups in similar reactive centres. However, selective protection/deprotection of orthogonal protecting groups in peptides is still challenging, especially for Cys-containing peptides, where protection of the cysteine side-chain is mandatory since the nucleophilic thiol can be otherwise alkylated, acylated or oxidized. Herein, we established a protocol for the synthesis of Cys-selective S-Trt or S-Mmt protected Cys-containing peptides, in a rapid way. This was achieved by, simply fine-tuning the carbocation scavenger in the final acidolytic release of the peptide from the solid support in the classic SPPS.








Similar content being viewed by others
References
Amand HL, Norden B, Fant K (2012) Functionalization with C-terminal cysteine enhances transfection efficiency of cell-penetrating peptides through dimer formation. Biochem Biophys Res Commun 418:469–474
Barlos K, Gatos D, Hatzi O, Koch N, Koutsogianni S (1996) Synthesis of the very acid-sensitive Fmoc-Cys(Mmt)-OH and its application in solid-phase peptide synthesis. Int J Pept Protein Res 47:148–153
Bauhuber S, Hozsa C, Breunig M, Gopferich A (2009) Delivery of nucleic acids via disulfide-based carrier systems. Adv Mater 21:3286–3306
Cremers CM, Jakob U (2013) Oxidant sensing by reversible disulfide bond formation. J Biol Chem 288(37):26489–26496
Fields C, Fields G (1993) Minimization of tryptophan alkylation following 9-fluorenylmethoxycarbonyl solid-phase peptide synthesis. Tetrahedron Lett 34:6661–6664
Fields GB, Noble RL (1990) Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res 35:161–214
Giraud M, Cavelier F, Martinez J (1999) A side-reaction in the SPPS of Trp-containing peptides. J Pept Sci 5:457–461
Góngora-Benítez M, Tulla-Puche J, Paradís-Bas M, Werbitzky O, Giraud M, Albericio F (2011) Optimized Fmoc solid-phase synthesis of the cysteine-rich peptide linaclotide. Biopolymers 96(1):69–80
Gongora-Benitez M, Basso A, Bruckdorfer T, Royo M, Tulla-Puche J, Albericio F (2012a) Eco-friendly combination of the immobilized PGA enzyme and the S-Phacm protecting group for the synthesis of Cys-containing peptides. Chem Eur J 18:16166–16176
Gongora-Benitez M, Mendive-Tapia L, Ramos-Tomillero I, Breman AC, Tulla-Puche J, Albericio F (2012b) Acid-labile Cys-protecting groups for the Fmoc/tBu strategy: filling the gap. Org Lett 14:5472–5475
Grundemann C, Thell K, Lengen K, Garcia-Kaufer M, Huang YH, Huber R et al (2013) Cyclotides suppress human T-lymphocyte proliferation by an interleukin 2-dependent mechanism. PLoS One 8:e68016
Hogg PJ (2013) Targeting allosteric disulphide bonds in cancer. Nat Rev Cancer 13:425–431
Karlstrom A, Rosenthal K, Unden A (2000) Study of the alkylation propensity of cations generated by acidolytic cleavage of protecting groups in Boc chemistry. J Pept Res 55:36–40
King DS, Fields CG, Fields GB (1990) A cleavage method which minimizes side reactions following Fmoc solid phase peptide synthesis. Int J Pept Protein Res 36:255–266
Lundt BF, Johansen NL, Volund A, Markussen J (1978) Removal of t-butyl and t-butoxycarbonyl protecting groups with trifluoroacetic acid. Mechanisms, biproduct formation and evaluation of scavengers. Int J Pept Protein Res 12:258–268
McCurdy SN (1989) The investigation of Fmoc-cysteine derivatives in solid phase peptide synthesis. Pept Res 2:147–152
McIntosh JM, Yoshikami D, Mahe E, Nielsen DB, Rivier JE, Gray WR et al (1994) A nicotinic acetylcholine receptor ligand of unique specificity, alpha-conotoxin ImI. J Biol Chem 269:16733–16739
Mehta A, Jaouhari R, Benson T, Douglas K (1992) Improved efficiency and selectivity in peptide synthesis: use of triethylsilane as a carbocation scavenger in deprotection of t-butyl esters and t-butoxycarbonyl-protected sites. Tetrahedron Lett 33:5441–5444
Papas S, Akoumianaki T, Kalogiros C, Hadjiarapoglou L, Theodoropoulos PA, Tsikaris V (2007) Synthesis and antitumor activity of peptide-paclitaxel conjugates. J Pept Sci 13:662–671
Pearson D, Blanchette M, Baker M, Guindon C (1989) Trialkylsilanes as scavengers for the trifluoroacetic acid deblocking of protecting groups in peptide synthesis. Tetrahedron Lett 30:2739–2742
Ramos-Tomillero I, Mendive-Tapia L, Gongora-Benitez M, Nicolas E, Tulla-Puche J, Albericio F (2013) Understanding acid lability of cysteine protecting groups. Molecules 18:5155–5162
Sarin VK, Kent SB, Tam JP, Merrifield RB (1981) Quantitative monitoring of solid-phase peptide synthesis by the ninhydrin reaction. Anal Biochem 117:147–157
Sieber P (1987) Modification of tryptophan residues during acidolysis of 4-methoxy-2,3,6-trimethylbenzenesulfonyl groups. Effects of scavengers. Tetrahedron Lett 28:1637–1640
Stathopoulos P, Papas S, Tsikaris V (2006) C-terminal N-alkylated peptide amides resulting from the linker decomposition of the Rink amide resin: a new cleavage mixture prevents their formation. J Pept Sci 12:227–232
Stathopoulos P, Papas S, Pappas C, Mousis V, Sayyad N, Theodorou V et al (2013) Side reactions in the SPPS of Cys-containing peptides. Amino Acids 44:1357–1363
Stierandova A, Sepetov NF, Nikiforovich GV, Lebl M (1994) Sequence-dependent modification of Trp by the Pmc protecting group of Arg during TFA deprotection. Int J Pept Protein Res 43:31–38
Torres AG, Gait MJ (2012) Exploiting cell surface thiols to enhance cellular uptake. Trends Biotechnol 30:185–190
Acknowledgments
The article is in line of a research project, co-financed by the European Union (European Regional Development Fund—ERDF) and Greek national funds through the Operational Program “Competitiveness and Entrepreneurship” of the National Strategic Reference Framework (NSRF)—Research Funding Program: Synergasia 2009. Action I. Cooperative small- and mid-scale projects (Project code: 09SYN-12-827). This research has been co-financed by the European Union (European Social Fund—ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF)—Research Funding Program: THALES Investing in knowledge society through the European Social Fund. (Acronym: FeRedox; ID: 1356). Moreover, special thanks are given to the Mass Spectrometry Unit of University of Ioannina, for providing access to the LC-MS/MS facilities.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Stathopoulos, P., Papas, S., Sakka, M. et al. A rapid and efficient method for the synthesis of selectively S-Trt or S-Mmt protected Cys-containing peptides. Amino Acids 46, 1367–1376 (2014). https://doi.org/10.1007/s00726-014-1696-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1696-0