Abstract
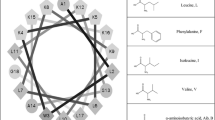
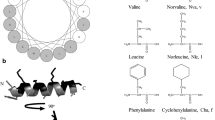
Antimicrobial peptides represent ancient host defense effector molecules present in organisms across the evolutionary spectrum. Lots of antimicrobial peptides were synthesized based on well-known structural motif widely existed in a variety of lives. Leucine-rich repeats (LRRs) are sequence motifs present in over 60,000 proteins identified from viruses, bacteria, and eukaryotes. To elucidate if LRR motif possesses antimicrobial potency, two peptides containing one or two LRRs were designed. The biological activity and membrane–peptide interactions of the peptides were analyzed. The results showed that the tandem of two LRRs exhibited similar antibacterial activity and significantly weaker hemolytic activity against hRBCs than the well-known membrane active peptide melittin. The peptide with one LRR was defective at antimicrobial and hemolytic activity. The peptide containing two LRRs formed α-helical structure, respectively, in the presence of membrane-mimicking environment. LRR-2 retained strong resistance to cations, heat, and some proteolytic enzymes. The blue shifts of the peptides in two lipid systems correlated positively with their biological activities. Other membrane-peptide experiments further provide the evidence that the peptide with two LRRs kills bacteria via membrane-involving mechanism. The present study increases our new understanding of well-known LRR motif in antimicrobial potency and presents a potential strategy to develop novel antibacterial agents.






Similar content being viewed by others
Abbreviations
- AMP:
-
Antimicrobial peptide
- LRR:
-
Leucine-rich repeat
- diSC3(5):
-
3-3-Dipropylthiadicarbocyanine-iodide
- MIC:
-
Minimum inhibitory concentration
- MHC:
-
Minimal hemolytic concentration
- CD:
-
Circular dichroism
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PG:
-
Phosphatidylglycerol
- NPN:
-
N-phenyl-1-naphthylamine
- MH:
-
Mueller–Hinton
References
Ahmad A, Yadav SP, Asthana N, Mitra K, Srivastava SP, Ghosh JK (2006) Utilization of an amphipathic leucine zipper sequence to design antibacterial peptides with simultaneous modulation of toxic activity against human red blood cells. J Biol Chem 281:22029–22038
Ahmad A, Azmi S, Ghosh JK (2011) Studies on the assembly of a leucine zipper antibacterial peptide and its analogs onto mammalian cells and bacteria. Amino Acids 40:749–759
Asthana N, Yadav SP, Ghosh JK (2004) Dissection of antibacterial and toxic activity of melittin. J Biol Chem 279:55042–55050
Bellaa J, Hindlea KL, McEwanb PA, Lovell SC (2008) The leucine-rich repeat structure. Cell Mol Life Sci 65:2307–2333
Bierne H, Sabet C, Personnic N, Cossart P (2007) Internalins: a complex family of leucine-rich repeat-containing proteins in Listeria monocytogenes. Microbes Infect 9:1156–1166
Brown KL, Hancock REW (2006) Cationic host defense (antimicrobial) peptides. Curr Opin Immunol 18:24–30
Chapman ER, Davis AF (1998) Direct interaction of a Ca2+-binding loop of synaptotagmin with lipid bilayers. J Biol Chem 273:13995–14001
DeYoung BJ, Innes RW (2006) Plant NBS-LRR proteins in pathogen sensing and host defense. Nat Immunol 7:1243–1249
Gooderham WJ, Bains M, McPhee JB, Wiegand I, Hancock REW (2008) Induction by cationic antimicrobial peptides and involvement in intrinsic polymyxin and antimicrobial peptide resistance, biofilm formation, and swarming motility of PsrA in Pseudomonas aeruginosa. J Bacteriol 190:5624–5634
Hasper HE, Kramer NE, Smith JL, Hillman JD, Zachariah C, Kuipers OP, de Kruijff B, Breukink E (2006) An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313:1636–1637
Huang HW (2000) Action of antimicrobial peptides: two-state model. Biochemistry 39:8347–8352
Ibrahim HR, Thomas U, Pellegrini A (2001) A helix-loop-helix peptide at the upper lip of the active site cleft of lysozyme confers potent antimicrobial activity with membrane permeabilization action. J Biol Chem 276:43767–43774
Kobayashi S, Chikushi A, Tougu S, Imura Y, Nishida M, Yano Y, Matsuzaki K (2004) Membrane translocation mechanism of the antimicrobial peptide buforin 2. Biochemistry 43:15610–15616
Ladokhin AS, White SH (2001) ‘Detergent-like’ permeabilization of anionic lipid vesicles by melittin. Biochim Biophys Acta 1514:253–260
Landschulz WH, Johnson PF, McKnight SL (1988) The leucine zipper: a hypothetical structure common to a new class of DNA-binding proteins. Science 240:1759–1764
Lee KH, Lee DG, Park Y, Kand DI, Shin SY, Hahm KS, Kim Y (2006) Interactions between the plasma membrane and the antimicrobial peptide HP (2–20) and its analogues derived from Helicobacter pylori. Biochem J 394:105–114
Loh B, Grant C, Hancock REW (1984) Use of the fluorescent probe 1-N-phenylnaphthylamine to study the interactions of aminoglycoside antibiotics with the outer membrane of Pseudomonas aeruginosa. Antimicrob Agents Chemother 26:546–551
Matsushima N, Kamiya M, Suzuki N, Tanaka T (2000) Super-motifs of leucine-rich repeats (LRRs) proteins. Genome Inform 11:343–345
Matsushima N, Miyashita H, Mikami T, Kuroki Y (2010) A nested leucine rich repeat (LRR) domain: the precursor of LRRs is a ten or eleven residue motif. BMC Microbiol 10:235–244
McHale L, Tan X, Koehl P, Michelmore RW (2006) Plant NBS-LRR proteins: adaptable guards. Genome Biol 7:212
Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A (2002) Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob Agents Chemother 46:689–694
Papo N, Shai Y (2003) Exploring peptide membrane interaction using surface plasmon resonance: differentiation between pore formation versus membrane disruption by lytic peptides. Biochemistry 42:458–466
Park S, Park SH, Ahn HC, Kim S, Kim SS, Lee BJ, Lee BJ (2001) Structural study of novel antimicrobial peptides, nigrocins, isolated from Rana nigromaculata. FEBS Lett 507:95–100
Park Y, Park SN, Park S, Shin SO, Kim J, Kang S, Kim M, Jeong C, Hahm K (2006) Synergism of Leu–Lys rich antimicrobial peptides and chloramphenicol against bacterial cells. Biochim Biophys Acta 1764:24–32
Pathak S, Chauhan VS (2011) Rationale-based, de novo design of dehydrophenylalanine-containing antibiotic peptides and systematic modification in sequence for enhanced potency. Antimicrob Agents Chemother 55:2178–2188
Rex S, Schwarz G (1998) Quantitative studies on the melittin-induced leakage mechanism of lipid vesicles. Biochemistry 37:2336–2345
Ryan CA, Huffaker A, Yamaguchi Y (2007) New insights into innate immunity in Arabidopsis. Cell Microbiol 9:1902–1908
Stark M, Liu LP, Deber CM (2002) Cationic hydrophobic peptides with antimicrobial activity. Antimicrob Agents Ch 46:3585–3590
Steinberg DA, Hurst MA, Fujii CA, Kung AHC, Ho JF, Cheng FC, Loury DJ, Fiddes JC (1997) Protegrin-1: a broad spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother 41:1738–1742
Wang G, Treleaven WD, Cushley RJ (1996) Conformation of human serum apolipoprotein A–I (166–185) in the presence of sodium dodecyl sulfate or dodecylphosphocholine by 1H-NMR and CD. Evidence for specific peptide-SDS interactions. Biochim Biophys Acta 1301:174–184
Wu M, Maier E, Benz R, Hancock REW (1999) Mechanism of Interaction of Different Classes of Cationic Antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli. Biochemistry 38:7235–7242
Yang ST, Shin SY, Lee CW, Kim YC, Hahm KS, Kim JI (2003) Selective cytotoxicity following Arg-to-Lys substitution in tritrpticin adopting a unique amphipathic turn structure. FEBS Lett 540:229–233
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55:27–55
Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415:389–395
Zelezetsky I, Tossi A (2006) Alpha-helical antimicrobial peptides–using a sequence template to guide structure-activity relationship studies. Biochim Biophys Acta 1758:1436–1449
Zhu WL, Song YM, Park Y, Park KH, Yang ST, Kim JI, Park IS, Hahm KS, Shin SY (2007) Substitution of the leucine zipper sequence in melittin with peptoid residues affects self-association, cell selectivity, and mode of action. Biochim Biophys Acta 1768:1506–1517
Acknowledgments
This work was supported by grants from the National Basic Research Program (2012CB124703), the National Natural Science Foundation of China (31072046 and 31272453), the Program for Innovative Research Team of Universities in Heilongjiang Province, the China Postdoctoral Science Foundation (2012M510082), and the Heilongjiang Postdoctoral Foundation (LBH-Z11238). We are pleased to thank Guo Hu, Xin Zhu, and Ze Y. Wang for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, Q.Q., Lv, Y.F., Gu, Y. et al. Rational design of cationic antimicrobial peptides by the tandem of leucine-rich repeat. Amino Acids 44, 1215–1224 (2013). https://doi.org/10.1007/s00726-012-1457-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1457-x