Abstract
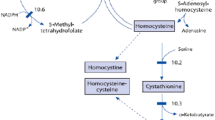
Homocysteine (Hcy) metabolites, Hcy-thiolactone and N-Hcy-proteins, have been linked to the pathology of human cardiovascular and neurodegenerative diseases. Hcy-thiolactone is generated in an error-editing reaction in protein biosynthesis when Hcy is selected in place of methionine by methionyl-tRNA synthetase. N-Hcy-protein, in which Hcy is linked via isopeptide bond to ε-amino group of a protein lysine residue, forms in a post-translational reaction of Hcy-thiolactone with proteins. Here, we identify a novel metabolite, Nε-Hcy-Lys, in human and mouse plasma, and show that this metabolite is elevated in genetic (cystathionine β-synthase deficiency in humans and mice, methylenetetrahydrofolate reductase deficiency in mice) or dietary (high Met diet in mice) deficiencies in Hcy metabolism. We also show that Nε-Hcy-Lys is generated by proteolytic degradation of N-Hcy-protein in mouse liver extracts. Our data indicate that free Nε-Hcy-Lys is an important pathology-related component of Hcy metabolism in humans and mice.




Similar content being viewed by others
References
Bald E, Chwatko G, Glowacki R, Kusmierek K (2004) Analysis of plasma thiols by high-performance liquid chromatography with ultraviolet detection. J Chromatogr A 1032:109–115
Chwatko G, Jakubowski H (2005a) The determination of homocysteine-thiolactone in human plasma. Anal Biochem 337:271–277
Chwatko G, Jakubowski H (2005b) Urinary excretion of homocysteine-thiolactone in humans. Clin Chem 51:408–415
Chwatko G, Boers GH, Strauss KA, Shih DM, Jakubowski H (2007) Mutations in methylenetetrahydrofolate reductase or cystathionine beta-synthase gene, or a high-methionine diet, increase homocysteine thiolactone levels in humans and mice. FASEB J 21:1707–1713
Daneshvar P, Yazdanpanah M, Cuthbert C, Cole DE (2003) Quantitative assay of plasma homocysteine thiolactone by gas chromatography/mass spectrometry. Rapid Commun Mass Spectrom 17:358–362
Ferretti G, Bacchetti T, Moroni C, Vignini A, Nanetti L, Curatola G (2004) Effect of homocysteinylation of low density lipoproteins on lipid peroxidation of human endothelial cells. J Cell Biochem 92:351–360
Glowacki R, Bald E (2009) Fully automated method for simultaneous determination of total cysteine, cysteinylglycine, glutathione and homocysteine in plasma by HPLC with UV absorbance detection. J Chromatogr B Analyt Technol Biomed Life Sci 877:3400–3404
Glowacki R, Jakubowski H (2004) Cross-talk between Cys34 and lysine residues in human serum albumin revealed by N-homocysteinylation. J Biol Chem 279:10864–10871
Gu W, Lu J, Yang G, Dou J, Mu Y, Meng J, Pan C (2008) Plasma homocysteine thiolactone associated with risk of macrovasculopathy in Chinese patients with type 2 diabetes mellitus. Adv Ther 25:914–924
Jakubowski H (1997) Metabolism of homocysteine thiolactone in human cell cultures. Possible mechanism for pathological consequences of elevated homocysteine levels. J Biol Chem 272:1935–1942
Jakubowski H (1999) Protein homocysteinylation: possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J 13:2277–2283
Jakubowski H (2000) Homocysteine thiolactone: metabolic origin and protein homocysteinylation in humans. J Nutr 130:377S–381S
Jakubowski H (2002a) The determination of homocysteine-thiolactone in biological samples. Anal Biochem 308:112–119
Jakubowski H (2002b) Homocysteine is a protein amino acid in humans. Implications for homocysteine-linked disease. J Biol Chem 277:30425–30428
Jakubowski H (2005) Anti-N-homocysteinylated protein autoantibodies and cardiovascular disease. Clin Chem Lab Med 43:1011–1014
Jakubowski H (2008) New method for the determination of protein N-linked homocysteine. Anal Biochem 380:257–261
Jakubowski H, Goldman E (1993) Synthesis of homocysteine thiolactone by methionyl-tRNA synthetase in cultured mammalian cells. FEBS Lett 317:237–240
Jakubowski H, Zhang L, Bardeguez A, Aviv A (2000) Homocysteine thiolactone and protein homocysteinylation in human endothelial cells: implications for atherosclerosis. Circ Res 87:45–51
Jakubowski H, Boers GH, Strauss KA (2008) Mutations in cystathionine beta-synthase or methylenetetrahydrofolate reductase gene increase N-homocysteinylated protein levels in humans. FASEB J 22:4071–4076
Jakubowski H, Perla-Kajan J, Finnell RH, Cabrera RM, Wang H, Gupta S, Kruger WD, Kraus JP, Shih DM (2009) Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J 23:1721–1727
Mudd SH, Finkelstein JD, Refsum H, Ueland PM, Malinow MR, Lentz SR, Jacobsen DW, Brattstrom L, Wilcken B, Wilcken DE, Blom HJ, Stabler SP, Allen RH, Selhub J, Rosenberg IH (2000) Homocysteine and its disulfide derivatives: a suggested consensus terminology. Arterioscler Thromb Vasc Biol 20:1704–1706
Mudd SH, Levy HL, Krauss JP (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B (eds) The metabolic and molecular bases of inherited disease, vol 2. Mc Graw-Hill, New York, pp 2007–2056
Paoli P, Sbrana F, Tiribilli B, Caselli A, Pantera B, Cirri P, De Donatis A, Formigli L, Nosi D, Manao G, Camici G, Ramponi G (2010) Protein N-homocysteinylation induces the formation of toxic amyloid-like aggregates. J Mol Biol (in press)
Perla-Kajan J, Marczak L, Kajan L, Skowronek P, Twardowski T, Jakubowski H (2007) Modification by homocysteine thiolactone affects redox status of cytochrome c. Biochemistry 46:6225–6231
Perla-Kajan J, Stanger O, Luczak M, Ziolkowska A, Malendowicz LK, Twardowski T, Lhotak S, Austin RC, Jakubowski H (2008) Immunohistochemical detection of N-homocysteinylated proteins in humans and mice. Biomed Pharmacother 62:473–479
Perna AF, Satta E, Acanfora F, Lombardi C, Ingrosso D, De Santo NG (2006) Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int 69:869–876
Sauls DL, Lockhart E, Warren ME, Lenkowski A, Wilhelm SE, Hoffman M (2006) Modification of fibrinogen by homocysteine thiolactone increases resistance to fibrinolysis: a potential mechanism of the thrombotic tendency in hyperhomocysteinemia. Biochemistry 45:2480–2487
Sibrian-Vazquez M, Escobedo JO, Lim S, Samoei GK, Strongin RM (2010) Homocystamides promote free-radical and oxidative damage to proteins. Proc Natl Acad Sci USA 107:551–554
Uji Y, Motomiya Y, Hanyu N, Ukaji F, Okabe H (2002) Protein-bound homocystamide measured in human plasma by HPLC. Clin Chem 48:941–944
Wang Z, Tang WH, Cho L, Brennan DM, Hazen SL (2009) Targeted metabolomic evaluation of arginine methylation and cardiovascular risks: potential mechanisms beyond nitric oxide synthase inhibition. Arterioscler Thromb Vasc Biol 29:1383–1391
Yang X, Gao Y, Zhou J, Zhen Y, Yang Y, Wang J, Song L, Liu Y, Xu H, Chen Z, Hui R (2006) Plasma homocysteine thiolactone adducts associated with risk of coronary heart disease. Clin Chim Acta Int J Clin Chem 364:230–234
Zang T, Dai S, Chen D, Lee BW, Liu S, Karger BL, Zhou ZS (2009) Chemical methods for the detection of protein N-homocysteinylation via selective reactions with aldehydes. Anal Chem 81:9065–9071
Acknowledgments
This work was supported in part by grants from the American Heart Association (0855919D) and the Ministry of Science and Higher Education, Poland (NN 401 230634, N401 065 32/1504, POIG.01.03.01-00-097/08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Głowacki, R., Bald, E. & Jakubowski, H. Identification and origin of Nε-homocysteinyl-lysine isopeptide in humans and mice. Amino Acids 39, 1563–1569 (2010). https://doi.org/10.1007/s00726-010-0627-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0627-y