Abstract
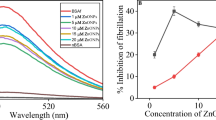
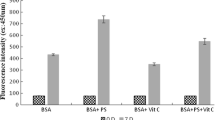
This work examines the effects of l-arginine (l-Arg) on the aggregation and amyloid fibrillation of bovine serum albumin (BSA). We demonstrate that l-Arg dose-dependently reduces thioflavin T (ThT) fluorescence of BSA within the l-Arg concentration range used (0–1.4 M). However, as revealed by electron microscopy, size exclusion chromatography, and dynamic light scattering results, l-Arg does not prevent amyloid-like fibril formation by BSA. We conclude that l-Arg competes against ThT for binding sites on BSA amyloid-like fibrils, leading to biased results in ThT fluorescence measurements. Moreover, the use of ThT fluorescence assay to screen for potential inhibitors against amyloid fibrillation can give misleading results.





Similar content being viewed by others
References
Arakawa T, Kita Y, Ejima D, Tsumoto K, Fukada H (2006) Aggregation suppression of proteins by arginine during thermal unfolding. Protein Pept Lett 13:921–927
Aso Y, Shiraki K, Takagi M (2007) Systematic analysis of aggregates from 38 kinds of non disease-related proteins: Identifying the intrinsic propensity of polypeptides to form amyloid fibrils. Biosci Biotechnol Biochem 71:1313–1321
Baynes BM, Wang DIC, Trout BL (2005) Role of arginine in the stabilization of proteins against aggregation. Biochemistry 44:4919–4925
Biancalana M, Makabe K, Koide A, Koide S (2009) Molecular mechanism of thioflavin-T binding to the surface of beta-rich peptide self-assemblies. J Mol Biol 385:1052–1063
Cardoso I, Goldsbury CS, Muller SA, Olivieri V, Wirtz S, Damas AM, Aebi U, Saraiva MJ (2002) Transthyretin fibrillogenesis entails the assembly of monomers: a molecular model for in vitro assembled transthyretin amyloid-like fibrils. J Mol Biol 317:683–695
Chiti F, Dobson CM (2006) Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 75:333–366
Chiti F, Dobson CM (2009) Amyloid formation by globular proteins under native conditions. Nat Chem Biol 5:15–22
Darghal N, Garnier-Suillerot A, Salerno M (2006) Mechanism of thioflavin T accumulation inside cells overexpressing P-glycoprotein or multidrug resistance-associated protein: role of lipophilicity and positive charge. Biochem Biophys Res Commun 343:623–629
De Bernardez Clark E (2001) Protein refolding for industrial processes. Curr Opin Biotech 12:202–207
Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15:3–16
Estrada LD, Soto C (2007) Disrupting beta-amyloid aggregation for Alzheimer disease treatment. Curr Top Med Chem 7:115–126
Ferrao-Gonzales AD, Souto SO, Silva JL, Foguel D (2000) The preaggregated state of an amyloidogenic protein: hydrostatic pressure converts native transthyretin into the amyloidogenic state. Proc Natl Acad Sci USA 97:6445–6450
Gazova Z, Bellova A, Daxnerova Z, Imrich J, Kristian P, Tomascikova J, Bagelova J, Fedunova D, Antalik M (2008) Acridine derivatives inhibit lysozyme aggregation. Eur Biophys J 37:1261–1270
Ghosh R, Sharma S, Chattopadhyay K (2009) Effect of arginine on protein aggregation studied by fluorescence correlation spectroscopy and other biophysical methods. Biochemistry 48:1135–1143
Gibson TJ, Murphy RM (2006) Inhibition of insulin fibrillogenesis with targeted peptides. Protein Sci 15:1133–1141
Goldsbury CS, Wirtz S, Muller SA, Sunderji S, Wicki P, Aebi U, Frey P (2000) Studies on the in vitro assembly of a beta 1–40: implications for the search for a beta fibril formation inhibitors. J Struct Biol 130:217–231
Groenning M, Olsen L, van de Weert M, Flink JM, Frokjaer S, Jorgensen FS (2007) Study on the binding of thioflavin T to beta-sheet-rich and non-beta-sheet cavities. J Struct Biol 158:358–369
Holm NK, Jespersen SK, Thomassen LV, Wolff TY, Sehgal P, Thomsen LA, Christiansen G, Andersen CB, Knudsen AD, Otzen DE (2007) Aggregation and fibrillation of bovine serum albumin. Biochim Biophys Acta-Proteins Proteomics 1774:1128–1138
Hudson SA, Ecroyd H, Kee TW, Carver JA (2009) The thioflavin T fluorescence assay for amyloid fibril detection can be biased by the presence of exogenous compounds. FEBS J 276:5960–5972
Ishibashi M, Tsumoto K, Tokunaga M, Ejima D, Kita Y, Arakawa T (2005) Is arginine a protein-denaturant? Protein Expr Purif 42:1–6
Khurana R, Coleman C, Ionescu-Zanetti C, Carter SA, Krishna V, Grover RK, Roy R, Singh S (2005) Mechanism of thioflavin T binding to amyloid fibrils. J Struct Biol 151:229–238
Krebs MRH, Bromley EHC, Donald AM (2005) The binding of thioflavin-T to amyloid fibrils: localisation and implications. J Struct Biol 149:30–37
Lai Z, Colon W, Kelly JW (1996) The acid-mediated denaturation pathway of transthyretin yields a conformational intermediate that can self-assemble into amyloid. Biochemistry 35:6470–6482
Lansbury PT Jr (1999) Evolution of amyloid: what normal protein folding may tell us about fibrillogenesis and disease. Proc Natl Acad Sci USA 96:3342–3344
LeVine H 3rd (1993) Thioflavine T interaction with synthetic Alzheimer’s disease beta- amyloid peptides: detection of amyloid aggregation in solution. Protein Sci 2:404–410
LeVine H (1999) Quantification of beta-sheet amyloid fibril structures with thioflavin T. Amyloid Prions Other Protein Aggreg 309:274–284
Lodderstedt G, Sachs R, Faust J, Bordusa F, Kuhn U, Golbik R, Kerth A, Wahle E, Balbach J, Schwarz E (2008) Hofmeister salts and potential therapeutic compounds accelerate in vitro fibril formation of the N-terminal domain of PABPN1 containing a disease-causing alanine extension. Biochemistry 47:2181–2189
Lyutova EM, Kasakov AS, Gurvits BY (2007) Effects of arginine on kinetics of protein aggregation studied by dynamic laser light scattering and turbidimetry techniques. Biotechnol Prog 23:1411–1416
Militello V, Vetri V, Leone M (2003) Conformational changes involved in thermal aggregation processes of bovine serum albumin. Biophys Chem 105:133–141
Morozova-Roche L, Malisauskas M (2007) A false paradise—mixed blessings in the protein universe: the amyloid as a new challenge in drug development. Curr Med Chem 14:1221–1230
Naiki H, Gejyo F (1999) Kinetic analysis of amyloid fibril formation. Methods Enzymol 309:305–318
Naiki H, Higuchi K, Hosokawa M, Takeda T (1989) Fluorometric-determination of amyloid fibrils invitro using the fluorescent dye, thioflavine-T. Anal Biochem 177:244–249
Pearce FG, Mackintosh SH, Gerrard JA (2007) Formation of amyloid-like fibrils by ovalbumin and related proteins under conditions relevant to food processing. J Agric Food Chem 55:318–322
Porat Y, Abramowitz A, Gazit E (2006) Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem Biol Drug Des 67:27–37
Reddy KR, Lilie H, Rudolph R, Lange C (2005) l-Arginine increases the solubility of unfolded species of hen egg white lysozyme. Protein Sci 14:929–935
Ross CA, Poirier MA (2004) Protein aggregation and neurodegenerative disease. Nat Med 10(Suppl):S10–S17
Serpell LC, Sunde M, Benson MD, Tennent GA, Pepys MB, Fraser PE (2000) The protofilament substructure of amyloid fibrils. J Mol Biol 300:1033–1039
Shtilerman MD, Ding TT, Lansbury PT Jr (2002) Molecular crowding accelerates fibrillization of alpha-synuclein: could an increase in the cytoplasmic protein concentration induce Parkinson’s disease? Biochemistry 41:3855–3860
Singh SM, Panda AK (2005) Solubilization and refolding of bacterial inclusion body proteins. J Biosci Bioeng 99:303–310
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68
Tsumoto K, Umetsu M, Kumagai I, Ejima D, Philo JS, Arakawa T (2004) Role of arginine in protein refolding, solubilization, and purification. Biotechnol Prog 20:1301–1308
Uversky VN, Fink AL (2004) Conformational constraints for amyloid fibrillation: the importance of being unfolded. Biochim Biophys Acta 1698:131–153
Vetri V, Librizzi F, Leone M, Militello V (2007) Thermal aggregation of bovine serum albumin at different pH: comparison with human serum albumin. Eur Biophys J 36:717–725
Wang W (2005) Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm 289:1–30
Wang SSS, Good TA (2005) An overview of Alzheimer’s disease. J Chin Inst Chem Eng 36:533–559
Wei Y, Chen L, Chen J, Ge L, He RQ (2009) Rapid glycation with d-ribose induces globular amyloid-like aggregations of BSA with high cytotoxicity to SH-SY5Y cells. BMC Cell Biol 10:10
Yamaguchi KI, Katou H, Hoshino M, Hasegawa K, Naiki H, Goto Y (2004) Core and heterogeneity of beta(2)-microglobulin amyloid fibrils as revealed by H/D exchange. J Mol Biol 338:559–571
Acknowledgments
This work was supported by grants from the National Science Council, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, KN., Wang, HY., Chen, CY. et al. l-Arginine reduces thioflavin T fluorescence but not fibrillation of bovine serum albumin. Amino Acids 39, 821–829 (2010). https://doi.org/10.1007/s00726-010-0536-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-010-0536-0